Get the Best Triflic Acid (CAS 1493-13-6) for Your Process – From Aure Chemical
Aure Chemical is a premier global supplier of high-quality Triflic Acid, formally known as Trifluoromethanesulfonic Acid (TFMSA). Identified by its CAS number 1493-13-6, Triflic Acid is one of the strongest commercially available Brønsted acids, widely recognized as a superacid. This colorless, hygroscopic liquid offers exceptional acidity, high thermal stability, and is non-oxidizing, making it an incredibly versatile and powerful reagent in numerous advanced chemical applications. Triflic Acid is indispensable as a highly efficient catalyst in complex organic synthesis, an effective electrolyte in demanding electrochemical systems, and a robust acid in the production of pharmaceuticals, agrochemicals, and specialty polymers. Aure Chemical is committed to providing Triflic Acid that meets stringent purity and performance requirements, ensuring consistent and reliable results for your most demanding industrial and research applications. Partner with us for a dependable supply of this critical chemical.
Basic Information of Triflic Acid
Triflic Acid (CAS No. 1493-13-6) is meticulously produced and rigorously tested to meet stringent quality standards. We offer various grades to suit your specific application requirements:
| CAS No.: | 1493-13-6 |
|---|
| EC No.: | 216-087-5 |
|---|
| Linear Formula: | CF₃SO₃H |
|---|
| Molecular Weight: | 150.08 |
|---|
| Appearance: | Colorless to slightly yellow liquid. |
|---|
| Melting Point: | -40 °C |
|---|
| Boiling point: | 162 °C (lit.) |
|---|
| Density: | 1.696 g/mL at 25 °C (lit.) |
|---|
| Solubility: | Miscible with water and many organic solvents. |
|---|
| Stability: | Highly stable thermally and hydrolytically; non-oxidizing. |
|---|
| Purity: | Available in high purity grades (e.g., 99% min) for sensitive catalytic and electrochemical applications. |
|---|
| RIDADR: | UN 3265 8/PG 2 |
|---|
| Chemical Structure: | ;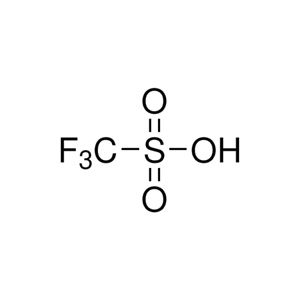 |
|---|
Our commitment to delivering high-purity Triflic Acid ensures a reliable and efficient component for your critical processes, offering consistent quality for diverse synthetic and industrial applications.
Primary Applications of Triflic Acid (Trifluoromethanesulfonic Acid)
Triflic Acid's extreme acidity and unique properties make it an invaluable reagent with significant applications in various advanced chemical processes:
Catalyst in Organic Synthesis:
As a powerful superacid, Triflic Acid is an exceptionally effective catalyst for a wide range of organic reactions. This includes:
Esterification and Amidation: Facilitates the formation of esters and amides under mild conditions.
Alkylation and Acylation: Highly efficient in Friedel-Crafts type reactions, promoting the addition of alkyl or acyl groups to aromatic compounds.
Polymerization: Used as an initiator or catalyst for the polymerization of various monomers, influencing molecular weight and polymer properties.
Isomerization and Rearrangement Reactions: Effective in facilitating complex molecular rearrangements.
Electrolyte in Electrochemical Applications:
Its high ionic conductivity and thermal stability make Triflic Acid a preferred electrolyte in advanced electrochemical systems, including certain types of fuel cells (e.g., proton exchange membrane fuel cells) and batteries, where its strong acidity and non-oxidizing nature are advantageous.
Pharmaceutical and Agrochemical Production:
Triflic Acid is a crucial reagent in the synthesis of complex active pharmaceutical ingredients (APIs) and agrochemicals. Its ability to catalyze highly selective reactions with high yields is invaluable in fine chemical manufacturing.
Precursor for Specialty Chemicals:
It serves as a key precursor for the synthesis of triflates (trifluoromethanesulfonate salts and esters), which are powerful leaving groups in organic synthesis and valuable intermediates in various chemical transformations.
Strong Acid Reagent:
Beyond its catalytic role, Triflic Acid is used as a potent strong acid in situations requiring extreme acidity, such as in the cleavage of protecting groups, or as a solvent for certain reactions.
Its role as one of the strongest non-oxidizing acids is discussed in detail in triflic acid as a superacid and activation reagent in organic synthesis.
Three Main Synthetic Routes
Electrolytic Fluorination Method:Methylsulfonyl chloride is electrolyzed in anhydrous hydrogen fluoride, where hydrogen atoms are gradually replaced by fluorine atoms. The final product is obtained and purified by distillation. This method requires sophisticated equipment but yields products of excellent purity.
Trifluoromethyl Oxidation Method:Using trifluoroiodomethane as the raw material, the reaction is catalyzed by a silver salt in the presence of sodium sulfite, followed by oxidation with hydrogen peroxide to obtain the target product. This route is suitable for small-scale laboratory synthesis.
Sulfur Trioxide Method:Trifluoromethane reacts directly with sulfur trioxide under the action of a catalyst. The process is relatively simple but requires strict temperature control (–30 °C to –10 °C).
Why Choose Aure Chemical for Your Triflic Acid Supply?
Aure Chemical is dedicated to providing superior chemical solutions and unparalleled customer support. By partnering with us for your Triflic Acid requirements, you benefit from:
Exceptional Purity & Consistent Quality: Our Triflic Acid is manufactured to stringent purity specifications, crucial for achieving optimal and reproducible results in sensitive catalytic processes, electrochemical research, and demanding synthetic reactions.
Reliable Global Supply Chain: We maintain a robust and efficient global supply network, guaranteeing timely and secure delivery of this essential superacid to your facilities worldwide, with flexible packaging options to meet your specific needs.
Expert Technical Support: Our dedicated team of specialists is readily available to offer comprehensive guidance on product application, safe handling, optimal storage, and usage in your specific chemical processes, ensuring safety and efficiency when working with a superacid.
Commitment to Quality & Responsible Stewardship: We adhere to the highest industry standards for quality management, environmental responsibility, and product stewardship across all our operations, ensuring peace of mind for our clients and sustainable sourcing practices.
Customized Solutions: We understand that different applications may require specific purity levels or concentrations. We are open to discussing customized solutions to meet your exact requirements.
Choose Aure Chemical for a trustworthy and dependable supply of high-quality Triflic Acid. We're ready to empower your most advanced chemical synthesis and industrial innovations with an unwavering commitment to quality and excellence.
Triflic Acid (CAS 1493-13-6) — FAQ
What is the pKa of Triflic Acid?
Triflic acid (CF₃SO₃H) is classified as a superacid with an estimated pKa of around –14.
This makes it much stronger than conventional strong acids such as sulfuric acid or hydrochloric acid.
What is the density of Triflic Acid?
The density of triflic acid is approximately 1.696 g/cm³ at 25 °C.
It is a colorless, fuming liquid that is highly corrosive and strongly hygroscopic.
Why is Triflic Acid hard to work with?
Triflic acid is extremely corrosive and reacts violently with water, releasing heat.
It requires specialized equipment (glass or PTFE-lined containers) and strict moisture control.
Handling should always be done in a fume hood with full PPE (acid-resistant gloves, goggles, and face protection).
How can Triflic Acid be distilled?
Triflic acid can be purified by vacuum distillation, typically using a glass apparatus with PTFE components.
The process must exclude moisture, as even trace water can cause violent reaction and decomposition.
Industrial-scale distillation should only be conducted by trained professionals under inert atmosphere.
Which is more acidic: Nitric Acid or Triflic Acid?
Triflic acid is much stronger than nitric acid.
Nitric acid has a pKa of about –1.4, while triflic acid’s pKa is about –14.
This large difference places triflic acid among the strongest known acids, often used in superacid chemistry.
For a broader understanding of how triflic acid relates to superacid behavior, triflation chemistry, and its derivatives, refer to the overview of triflic acid and triflate chemistry.
Hazards Classification
GHS Classification: Corrosive (GHS05), Acute Toxicity (GHS06), Exclamation Mark (GHS07)
Hazard Statements: Causes severe skin burns and eye damage; harmful if swallowed or inhaled; may cause respiratory irritation.
UN Number: UN 3265
Hazard Class: 8 (Corrosive)
Packing Group: II
 GHS05: Corrosive
GHS05: Corrosive GHS06: Acute toxicity
GHS06: Acute toxicity GHS07: Exclamation mark
GHS07: Exclamation mark
