How does Triflic Acid undergo dehydration to form Triflic Anhydride?
Trifluoromethanesulfonic acid (CF₃SO₃H, commonly known as Triflic Acid) is one of the strongest known Brønsted acids, with a pK_a of approximately -14, making it highly useful in organic synthesis as a catalyst or reagent. However, its dehydration to form trifluoromethanesulfonic anhydride ((CF₃SO₂)₂O, or Triflic Anhydride) is a key transformation, as the anhydride is a versatile electrophile for introducing triflate groups in reactions like triflation of alcohols, ketones, or amides. The dehydration process removes water from two molecules of triflic acid to yield the anhydride:
2CF₃SO₃H →(CF₃SO₂)₂O+H₂O
This reaction is endothermic and requires a dehydrating agent to drive it forward, as triflic acid is hygroscopic and stable in aqueous environments. Two common dehydrating agents are phosphorus pentoxide (P₄O₁₀, often referred to as P₂O₅) and ketene (CH₂=C=O) or its derivatives. Below is a detailed analysis of each method, including mechanisms, reaction conditions, yields, advantages, limitations, and practical considerations.
Dehydration with Phosphorus Pentoxide (P₄O₁₀)
Reaction and Conditions
The use of P₄O₁₀ for dehydrating triflic acid is a classical method, leveraging its strong affinity for water to shift the equilibrium toward the anhydride. The overall reaction involves mixing triflic acid with excess P₄O₁₀, followed by stirring and distillation to isolate the product. A typical procedure involves creating a slurry of P₄O₁₀ (e.g., 3.2 g) in crude triflic anhydride (31.2 g) and stirring at room temperature for 18 hours in a stoppered flask. The anhydride is then distilled under reduced pressure to avoid decomposition.
Molar Ratio: P₄O₁₀ to triflic acid is typically in excess (e.g., 1:2 or higher) to ensure complete water removal.
Temperature: Room temperature (20-25°C) for stirring; distillation at 80-100°C under vacuum (e.g., 12 mmHg or 1600 Pa) to collect the fraction boiling at ~76°C.
Solvent: Often solvent-free or in inert media like the crude anhydride itself to minimize side reactions.
Scale-Up Tips: For larger scales, premixing P₄O₁₀ with an equal volume of Celite (filter aid) improves yields and ease of handling by preventing clumping.
Yields: High yields (up to 90-95%) are achievable with pure starting materials, though impurities in the acid can reduce efficiency. Patents report efficient production with optimized ratios.
Mechanism
P₄O₁₀ acts as a dehydrating agent by reacting with the water liberated from the acid, forming phosphoric acid derivatives. The process begins with the equilibrium dehydration of triflic acid, which is slow due to the acid's stability:
2CF₃SO₃H ⇌ (CF₃SO₂)₂O+H₂O
P₄O₁₀ irreversibly consumes the water:
P3O10+6H2O→4H3PO4
This shifts the equilibrium rightward. Mechanistically, P₄O₁₀'s electrophilic phosphorus atoms attack the oxygen of water, forming metaphosphates or orthophosphates as by-products. The reaction is heterogeneous (slurry), with stirring ensuring contact. No catalysts are needed, but the excess P₄O₁₀ also helps dry any residual moisture in the acid.
Advantages and Limitations
Advantages: Simple, cost-effective, and widely used for laboratory and industrial scales. Produces high-purity anhydride after distillation. Effective for purifying crude triflic acid by removing water and other volatiles.
Limitations: Generates non-recyclable phosphorus-containing waste (e.g., H₃PO₄), posing environmental concerns. The reaction can be exothermic if not controlled, and excess P₄O₁₀ may require filtration. Not ideal for continuous processes due to solid handling.
Dehydration with Ketene
Reaction and Conditions
Ketene (or substituted ketenes like dimethylketene) provides a phosphorus-free alternative, proceeding via formation of a mixed anhydride intermediate, followed by disproportionation during distillation. The process is particularly advantageous for industrial applications, as it avoids waste and catalysts.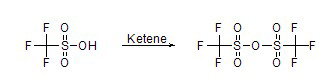
Reaction Equation: Using dimethylketene as an example:
Formation of mixed anhydride: CF₃SO₃H + (CH₃)₂C=C=O → CF₃SO₂—O—C(O)—CH(CH₃)₂ (isobutyryl triflate).
Disproportionation: 2 CF₃SO₂—O—C(O)—R → (CF₃SO₂)₂O + (RCO)₂O, where R = CH(CH₃)₂ for isobutyric anhydride.
Conditions:
Mixed Anhydride Formation: Temperature -20 to 40°C (range -76 to 70°C); mole ratio triflic acid to ketene 10:1 to 0.9:1; ambient pressure; optional chlorinated solvent (e.g., dichloroethane), though solvent-free preferred.
Disproportionation via Reactive Distillation: Column head 75-85°C, base 170-190°C for dimethylketene (adjustable for other ketenes); reduced pressure to keep base <160°C; continuous or semi-continuous mode.
Yields: High (not quantified in percentages, but described as efficient with near-complete conversion); slight ketene excess ensures purity.
Scale: Suitable for large-scale production due to rapid reaction and minimal by-products.
Mechanism
The reaction occurs in two steps:
Nucleophilic Addition to Form Mixed Anhydride: The oxygen of triflic acid's OH group attacks the electrophilic carbon of ketene's C=O, with the ketene's β-carbon accepting a proton, forming the mixed anhydride (CF₃SO₂—O—C(O)—CR₂). This is rapid and exothermic, requiring no catalyst due to triflic acid's strength.
Disproportionation: In the distillation column, the mixed anhydride undergoes thermal redistribution, where two molecules exchange groups to form symmetric anhydrides. This is facilitated by multiple vapor-liquid equilibria in the column, driving separation: triflic anhydride (b.p. ~82°C) distills overhead, while the higher-boiling carboxylic anhydride (e.g., isobutyric anhydride, b.p. ~182°C) remains at the base.
Advantages and Limitations
Advantages: Phosphorus-free, producing recyclable carboxylic anhydrides as by-products; rapid, catalyst-free, and environmentally friendlier. Adaptable to continuous processes with high selectivity.
Limitations: Requires careful temperature control to avoid side reactions (e.g., polymerization); ketene handling is hazardous (toxic, reactive gas), necessitating specialized equipment. Less common in labs due to ketene availability.
Comparison and Practical Considerations
Efficiency and Purity: P₄O₁₀ is simpler for small-scale but wasteful; ketene offers cleaner, scalable production with better yields in industrial settings.
Environmental Impact: Ketene method minimizes waste, aligning with green chemistry; P₄O₁₀ generates phosphoric residues requiring disposal.
Cost and Safety: P₄O₁₀ is cheaper but corrosive; ketene is efficient but demands safety protocols for its flammability and toxicity.
Applications: Both yield high-purity triflic anhydride (>99%), used in synthesis. Choose based on scale: P₄O₁₀ for batch, ketene for continuous.
In practice, handle triflic acid under inert atmospheres to avoid moisture, and distill products under vacuum for safety.

