Aure Chemical: Your Reliable Partner for High-Quality Boron Trifluoride Methanol Complex
Aure Chemical is a trusted supplier of Boron Trifluoride Methanol Complex, a highly effective and versatile Lewis acid catalyst. This clear, colorless liquid provides a convenient and controlled source of Boron Trifluoride (BF₃), making it an invaluable reagent for a wide array of organic synthesis applications. Our Boron Trifluoride Methanol Complex is manufactured to high purity standards, ensuring optimal performance for your most critical chemical reactions.
Basic Information of Boron Trifluoride Methanol Complex
Boron Trifluoride Methanol Complex (CAS No. 2802-68-8) is characterized by its significant properties:
| CAS No.: | 2802-68-8 |
|---|
| EC No.: | 220-543-9 |
|---|
| Linear Formula: | C₂H₈BF₃O₂ |
|---|
| Molecular Weight: | 131.89 |
|---|
| Appearance: | Colorless to light orange to yellow clear liquid |
|---|
| Melting point: | -20 °C |
|---|
| Boiling point: | 58-60°C 4mm |
|---|
| Density: | 1.22-1.25 g/mL at 20 °C |
|---|
| Solubility | Miscible with alcohols, ethers, esters; reacts exothermically with water. |
|---|
| Flash Point: | 64 °C |
|---|
| Nature: | Strong Lewis acid. |
|---|
| RIDADR: | UN 2924 6.1/PG 3 |
|---|
| Chemical Structure: | 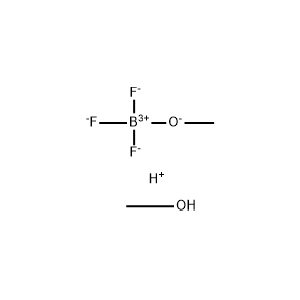 |
|---|
These attributes make it a preferred catalytic system in processes where a controlled acidic environment is required.
Main Functions and Applications of Boron Trifluoride Methanol Complex
The catalytic power and unique reactivity of Aure Chemical's Boron Trifluoride Methanol Complex enable its diverse applications:
As an Esterification Reagent for Acids: The boron trifluoride methanol complex serves as an effective acidic reagent to promote esterification reactions, particularly for the synthesis of methyl esters.
For the Selective Cleavage of Ethers: In organic synthesis, it can be used for the selective cleavage of various ether linkages under specific conditions, offering a valuable tool for complex molecule synthesis.
Deconjugation of Acetylamides in Anthocyanin Dyes: Boron trifluoride methanol complexes can also be utilized in specialized applications, such as for the deconjugation of acetylamides in anthocyanin dyes, yielding products with free amino groups.
Lewis Acid and Catalyst in Organic Synthesis: Boron trifluoride methanol solution is commonly used as a highly effective Lewis acid catalyst in organic chemistry, particularly in promoting and accelerating a wide range of organic reactions beyond esterification.
Olefin Polymerization Field: This complex plays a significant role in the olefin polymerization field. For example, it is used as a key catalyst in the synthesis of polyalphaolefins (PAO), which serve as crucial lubricating oil base stocks.
Why Choose Aure Chemical for Your Boron Trifluoride Methanol Complex Needs?
Aure Chemical is committed to delivering excellence and reliability in chemical supply. When you choose us for your Boron Trifluoride Methanol Complex requirements, you benefit from:
Assured Purity & Performance: Our product undergoes stringent quality control to ensure high purity and consistent catalytic activity, vital for sensitive chemical processes.
Reliable Supply Chain: We maintain a robust and efficient global supply chain, ensuring timely and secure delivery of this valuable reagent.
Technical Expertise: Our knowledgeable team is always available to provide comprehensive support and answer any technical questions you may have regarding product application, handling, and safety.
Commitment to Quality & Safety: We adhere to the highest quality control, safety, and environmental standards throughout our operations.
Partner with Aure Chemical for a seamless and dependable supply of high-quality Boron Trifluoride Methanol Complex. We are ready to assist you in finding the perfect solution for your specific application requirements.
Hazards Classification
GHS Classification:
Skin Corr. / Eye Damage (GHS05), Acute Toxicity — oral/inhalation/dermal (GHS06), Specific target organ toxicity — single exposure (GHS08), Flammable Liquid (GHS02)
Hazard Statements:
Causes severe skin burns and eye damage (H314); toxic if swallowed, inhaled or in contact with skin (H301, H331, H311); causes damage to organs (H370); highly flammable or combustible depending on concentration (H225 / combustible liquid statement).
UN Number: UN 2924
Hazard Class: 8 (Corrosive substances); Subsidiary hazard class: 6.1 (Toxic substances)
Packing Group: I
 GHS05: Corrosive (skin corrosion / eye damage)
GHS05: Corrosive (skin corrosion / eye damage) GHS06: Acute Toxicity
GHS06: Acute Toxicity GHS08: STOT (organ damage)
GHS08: STOT (organ damage) GHS02: Flammable (when concentration/mixture contains sufficient methanol)
GHS02: Flammable (when concentration/mixture contains sufficient methanol)
