Aure Chemical Delivers Excellence in Triallylamine
Triallylamine (CAS 102-70-5) is a tertiary amine containing three allyl groups, making it highly suitable for
applications requiring multifunctional allylic reactivity. It is commonly used in the manufacture of specialty
monomers, crosslinking agents, polymer-modification intermediates, and fine chemicals. Its structure supports
controlled polymerization, grafting reactions, and advanced synthetic transformations. Aure Chemical supplies
consistent-quality grades suitable for industrial production, R&D, and custom synthesis projects.
Basic Information of Triallylamine
Meticulously produced and rigorously tested to meet stringent quality standards. We ensure exceptional purity and consistent performance, essential for your critical applications:
| CAS No.: | 102-70-5 |
|---|
| EC No.: | 203-048-2 |
|---|
| Linear Formula: | C9H15N |
|---|
| Molecular Weight: | 137.22 |
|---|
| Appearance: | Colorless to light yellow liquid |
|---|
| Odor: | Characteristic amine odor |
|---|
| Melting Point: | -70°C |
|---|
| Boiling Point: | 150-151 °C (lit.) |
|---|
| Density: | 0.79 g/mL at 25 °C (lit.) |
|---|
| Solubility: | Soluble in common organic solvents; limited solubility in water |
|---|
| Flash Point: | 87 °F |
|---|
| RIDADR: | UN 2610 3/PG 3 |
|---|
| Chemical Structure: | 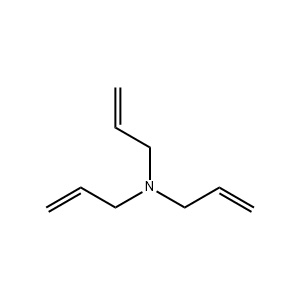 |
|---|
Application Overview
Triallylamine is valued as a multi-functional allylic amine that supports various industrial and synthetic
pathways. Its three allyl groups enable high reactivity and versatility in fields involving polymer
modification, monomer design, and advanced organic synthesis. Major uses include:
Specialty Monomers & Polymer Crosslinkers: Ideal for synthesizing triallyl derivatives used to increase crosslink density, improve mechanical strength, and tailor polymer network structures.
Resins & Thermosetting Materials: A building block for producing heat-resistant resins, UV-curable systems, and advanced composites.
Fine Chemical & Intermediate Synthesis: Useful in allylic substitution reactions, catalytic transformations, and amine-functional derivative production.
Coatings, Adhesives & Sealants: Supports development of performance additives that enhance adhesion, chemical resistance and curing behavior.
Electronic & Material Applications: Used in formulations for photoresists, ion-exchange materials, and functional polymers requiring reactive allyl groups.
Research & Innovation Projects: Common in polymer design studies, crosslinker development, and specialty R&D chemistry due to its high functional density.
Aure Chemical provides technical documentation, data consistency, and impurity insights to help integrate
triallylamine into both established and exploratory synthesis processes.
Why Choose Aure Chemical
Aure Chemical ensures dependable sourcing and technical partnership for customers using triallylamine:
Reliable Global Supply: Long-term procurement contracts and diversified sourcing help maintain consistent product availability.
Strict Quality Assurance: Each batch undergoes analytical testing to ensure purity, stability, and reproducible reaction performance.
Technical Collaboration: Support teams assist with reaction optimization, impurity control, and integration into process workflows.
Flexible Fulfillment: Options from small R&D quantities to bulk industrial shipments, with custom packaging upon request.
Regulatory & Documentation Support: SDS, COA, transportation classification, and export documents are provided to simplify cross-border operations.
Customer-Centered Logistics: Efficient shipment coordination, consolidated orders, and dependable lead times help streamline supply chains.
Hazards Classification
GHS Classification: Flammable Liquid (GHS02), Acute Toxicity (GHS06), Corrosive (GHS05)
Hazard Statements: Highly flammable liquid and vapor; toxic if swallowed, in contact with skin or if inhaled; causes severe skin burns and eye damage.
UN Number: UN 2610
Hazard Class: 3 (Flammable Liquids)
Packing Group: III
 GHS02: Flammable
GHS02: Flammable GHS05: Corrosive
GHS05: Corrosive GHS06: Acute toxicity
GHS06: Acute toxicity
