High-Purity Silver Perchlorate from Aure Chemical – Your Trusted Source
Silver perchlorate, AgClO₄, CAS No. 7783-93-9, is a highly reactive and strongly oxidizing silver(I) salt renowned for its exceptional solubility in both water and organic solvents. Supplied by Aure Chemical as white to off-white crystalline powder or hydrated solid with high silver content (typically 50–52% Ag anhydrous basis), this extremely hygroscopic compound serves as one of the most powerful sources of naked Ag⁺ ions due to the very low coordinating ability of the perchlorate anion. It is widely used as a strong Lewis acid in organic synthesis, precursor for silver nanoparticle and colloid preparation, synthesis of organometallic silver complexes, and in applications requiring high Ag⁺ activity (e.g., halide abstraction, catalysis, and coordination chemistry). Its anhydrous form is particularly valued for non-aqueous systems where maximum electrophilicity is required. Aure Chemical provides controlled hydration options, precise Ag assay, low residual impurities, and secure silver sourcing to ensure batch consistency and optimal performance in demanding research and industrial applications worldwide.
Basic Information of Silver Perchlorate
Aure Chemical supplies Silver Perchlorate as a premium Ag(I) precursor with verified high silver content and exceptional purity for Lewis acid catalysis and synthesis applications.
| CAS No. | 7783-93-9 (anhydrous); hydrate forms also available |
|---|
| EC No. | 232-032-9 |
|---|
| Chemical Formula | AgClO₄ (anhydrous) |
|---|
| Molecular Weight | 207.32 g/mol (anhydrous) |
|---|
| Appearance | White to off-white crystalline powder (anhydrous); hydrated forms may appear slightly yellow |
|---|
| Odor | Odorless |
|---|
| Melting point | 486 °C (anhydrous, decomposes) |
|---|
| Boiling point | Not applicable (decomposes) |
|---|
| Density | approx. 2.8 g/cm³ (anhydrous) |
|---|
| Solubility | Extremely soluble in water (>500 g/100 mL); highly soluble in organic solvents (acetonitrile, DMSO, DMF, benzene, toluene) |
|---|
| Nature (hazards) | Strong oxidizer, explosive when mixed with organics, causes severe skin burns and eye damage, harmful if swallowed or inhaled, toxic to aquatic life |
|---|
| RIDADR | UN 1481, Oxidizing solid, n.o.s. (Silver perchlorate), Class 5.1, Packing Group II |
|---|
| Chemical Structure | 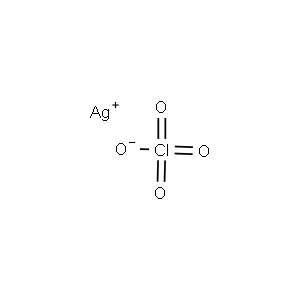 |
|---|
Aure Chemical offers both anhydrous and hydrated forms, customizable silver content, purity grades, and specialized packaging (light- and moisture-protected) to meet your catalysis, nanoparticle, or synthesis requirements.
Primary Applications of Silver Perchlorate
Silver Perchlorate is one of the strongest sources of "naked" Ag⁺ and is prized for its powerful Lewis acidity and solubility in organic media.
Strong Lewis Acid Catalysis
Used in organic synthesis for halide abstraction, activation of carbonyls, alkynes, and alkenes, and as a co-catalyst in gold/silver-mediated reactions requiring high Ag⁺ electrophilicity.
Silver Nanoparticle & Colloid Synthesis
Employed in reduction processes (especially in organic solvents) to generate Ag nanoparticles with unique electronic properties for plasmonics, SERS, and catalysis.
Organometallic Silver Complex Synthesis
Preferred precursor for preparing Ag(I) complexes with phosphines, carbenes, or weakly coordinating ligands due to minimal interference from the perchlorate anion.
Antimicrobial & Biocidal Applications
Provides highly available Ag⁺ for antimicrobial coatings, textiles, and medical devices where rapid silver ion release is desired.
Supported Silver Catalyst Preparation
Used in impregnation or deposition to create highly active Ag catalysts on supports for selective oxidation and other redox processes.
Advanced Materials & Electrochemistry
Employed in silver perchlorate-based electrolytes, reference electrodes, and functional materials requiring high Ag⁺ activity.
Why Choose Aure Chemical for Your Silver Perchlorate Supply?
Select Aure Chemical as your reliable partner for Silver Perchlorate, delivering exceptional reactivity, technical support, and stable silver supply for demanding applications.
Superior Purity & Anhydrous Options
Rigorous quality controls ensure high Ag content, minimal chloride/heavy metal impurities, and availability of anhydrous material for maximum Lewis acidity.
Secure Precious Metal Sourcing
Transparent, responsible silver procurement with inventory buffering to provide supply reliability and competitive pricing.
Technical Catalysis Support
Experienced specialists offer guidance on handling, activation, reduction protocols, and reaction optimization for maximum efficiency.
Comprehensive Regulatory Compliance
Full COA, REACH, RoHS, and detailed SDS documentation to facilitate seamless integration into global operations and audits.
Sustainability & Recycling Programs
Ethical sourcing practices and silver recovery/recycling programs aligned with ESG objectives and circular economy principles.
Hazards Classification
GHS Classification: Oxidizing Solids (Category 2); Skin Corrosion (Category 1B); Serious Eye Damage (Category 1); Acute Toxicity, Oral (Category 4); Aquatic Chronic (Category 2)
Hazard Statements: H272: May intensify fire; oxidizer; H314: Causes severe skin burns and eye damage; H302: Harmful if swallowed; H411: Toxic to aquatic life with long lasting effects
UN Number: UN 1481
Hazard Class: 5.1 (Oxidizing substances)
Packing Group: II
 GHS03: Oxidizer
GHS03: Oxidizer GHS05: Corrosive
GHS05: Corrosive GHS07: Exclamation mark (acute toxicity)
GHS07: Exclamation mark (acute toxicity) GHS09: Environment (aquatic toxicity)
GHS09: Environment (aquatic toxicity)
