High-Purity Silver(I) Fluoride from Aure Chemical – Your Trusted Source
Silver(I) fluoride, AgF, CAS No. 7775-41-9, is a highly reactive silver(I) halide widely recognized for its exceptional solubility in water and unique applications in fluorination chemistry and materials science. Supplied by Aure Chemical as fine white to pale yellow crystalline powder with high silver content (approximately 85–86% Ag), this hygroscopic solid serves as an efficient source of Ag⁺ and F⁻ ions. It is the preferred reagent for nucleophilic fluorination of organic halides, synthesis of fluorinated organometallic complexes, preparation of silver fluoride-based catalysts, and generation of silver nanoparticles with fluoride interfaces. Its strong fluoride-donating ability and ability to form stable Ag–F bonds make it indispensable in pharmaceutical fluorination, agrochemical synthesis, and advanced functional materials. Aure Chemical provides controlled purity, low chloride/heavy metal impurities, and secure silver sourcing to ensure batch consistency and optimal performance in research and industrial applications worldwide.
Basic Information of Silver(I) Fluoride
Aure Chemical supplies Silver(I) Fluoride as a premium Ag(I) precursor with verified high silver content and exceptional purity for fluorination and materials applications.
| CAS No. | 7775-41-9 |
|---|
| EC No. | 231-895-8 |
|---|
| Chemical Formula | AgF |
|---|
| Molecular Weight | 126.87 g/mol |
|---|
| Appearance | Fine white to pale yellow crystalline powder |
|---|
| Odor | Odorless |
|---|
| Melting point | 435 °C |
|---|
| Boiling point | ~1150 °C (decomposes) |
|---|
| Density | 5.852 g/cm³ |
|---|
| Solubility | Highly soluble in water (~172 g/100 mL at 20 °C); soluble in ammonia, dilute acids |
|---|
| Nature (hazards) | Causes severe skin burns and eye damage, harmful if swallowed or inhaled, toxic to aquatic life |
|---|
| RIDADR | UN 3260, Corrosive solid, acidic, inorganic, n.o.s. (Silver fluoride), Class 8, Packing Group II |
|---|
| Chemical Structure | 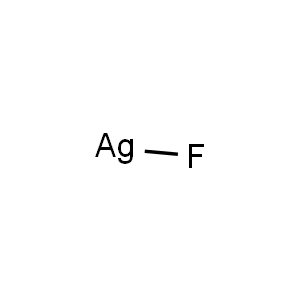 |
|---|
Aure Chemical offers customizable options including silver content specification (85–86% Ag typical), purity grades, particle size control, and moisture-protected packaging to meet your fluorination, nanoparticle, or synthesis requirements.
Primary Applications of Silver(I) Fluoride
Silver(I) Fluoride is a powerful Ag⁺ and F⁻ source, widely used in fluorination chemistry and silver-based materials.
Nucleophilic Fluorination
Serves as the reagent of choice for halogen exchange (Finkelstein-type) reactions, converting alkyl chlorides/bromides/iodides to fluorides under mild conditions in pharmaceutical and agrochemical synthesis.
Organometallic Silver Complex Synthesis
Acts as precursor for preparing Ag(I) fluoride complexes and fluorinated organometallic species in coordination chemistry and catalysis research.
Silver Nanoparticle & Colloid Synthesis
Used in reduction processes to generate Ag nanoparticles with fluoride interfaces, useful in plasmonics, SERS, and antimicrobial applications.
Supported Silver Catalyst Preparation
Employed in impregnation or reduction to deposit Ag on supports for selective oxidation, ethylene oxide catalysis, and fluorination-related processes.
Antimicrobial & Biocidal Applications
Provides Ag⁺ release for antimicrobial coatings, textiles, and medical devices, enhanced by fluoride synergy in certain formulations.
Advanced Materials & Electrochemistry
Used in silver fluoride thin films, solid electrolytes, and functional materials requiring fluoride-containing silver species.
Why Choose Aure Chemical for Your Silver(I) Fluoride Supply?
Partner with Aure Chemical for Silver(I) Fluoride to access premium fluorination reactivity, technical expertise, and stable precious metal supply for your silver-based applications.
Accurate Silver Content & Purity
Precise analytical control ensures uniform Ag content, minimal chloride/heavy metal impurities, and batch reproducibility for reliable performance.
Secure Precious Metal Sourcing
Transparent, responsible silver procurement with inventory buffering to provide supply stability and competitive pricing.
Technical Application Support
Dedicated specialists provide advice on fluorination protocols, nanoparticle reduction, catalyst preparation, and process optimization.
Full Regulatory Compliance
Comprehensive COA, REACH, RoHS, and detailed SDS documentation to facilitate global operations and audits.
Sustainability & Recycling Commitment
Ethical sourcing practices and silver recovery/recycling programs aligned with ESG objectives and circular economy principles.
Hazards Classification
GHS Classification: Skin Corrosion (Category 1B); Serious Eye Damage (Category 1); Acute Toxicity, Oral (Category 4); Acute Toxicity, Inhalation (Category 4); Aquatic Chronic (Category 2)
Hazard Statements: H314: Causes severe skin burns and eye damage; H302 + H332: Harmful if swallowed or if inhaled; H411: Toxic to aquatic life with long lasting effects
UN Number: UN 3260
Hazard Class: 8 (Corrosive substances)
Packing Group: II
 GHS05: Corrosive
GHS05: Corrosive GHS07: Exclamation mark (acute toxicity)
GHS07: Exclamation mark (acute toxicity) GHS09: Environment (aquatic toxicity)
GHS09: Environment (aquatic toxicity)
