High-Purity Potassium Tetrachloroplatinate(II) from Aure Chemical – Your Trusted Source
Potassium tetrachloroplatinate(II), K₂[PtCl₄], CAS No. 10025-99-7, is a cornerstone anionic platinum(II) complex widely recognized as a key precursor in coordination and medicinal chemistry. Supplied by Aure Chemical as reddish-brown to dark red crystalline powder with high platinum content (approximately 44–46% Pt), this water-soluble salt provides a stable, readily available source of [PtCl₄]²⁻ ions. It plays a central role in the synthesis of cisplatin and other platinum-based anticancer agents, preparation of organometallic Pt(II) complexes, ligand substitution reactions, and generation of platinum catalysts or nanoparticles. Its well-defined structure, high purity, and controlled reactivity make it indispensable for pharmaceutical manufacturing, academic research, and advanced materials development. Aure Chemical delivers precise Pt assay, low impurity levels, and secure platinum sourcing to ensure reproducible, high-performance results in global applications.
Basic Information of Potassium Tetrachloroplatinate(II)
Aure Chemical offers Potassium Tetrachloroplatinate(II) as a premium-grade anionic Pt(II) precursor with verified platinum content and exceptional purity for synthesis and research applications.
| CAS No. | 10025-99-7 |
|---|
| EC No. | 233-050-9 |
|---|
| Chemical Formula | K2PtCl4 |
|---|
| Molecular Weight | 415.09 g/mol |
|---|
| Appearance | Reddish-brown to dark red crystalline powder |
|---|
| Odor | Odorless |
|---|
| Melting point | Decomposes before melting (>300 °C) |
|---|
| Boiling point | Not applicable (decomposes) |
|---|
| Density | approx. 3.50 g/cm³ |
|---|
| Solubility | Soluble in water (~50 g/L at 20 °C); slightly soluble in ethanol; insoluble in most organic solvents |
|---|
| Nature (hazards) | Causes severe skin burns and eye damage, may cause allergic skin reaction, harmful if swallowed, toxic to aquatic life with long lasting effects |
|---|
| RIDADR | UN 3288, Toxic solid, inorganic, n.o.s. (Potassium tetrachloroplatinate(II)), Class 6.1, Packing Group III |
|---|
| Chemical Structure | 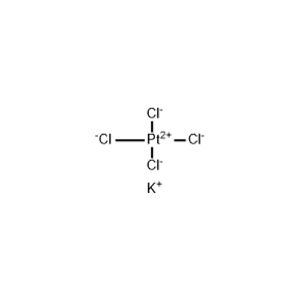 |
|---|
Aure Chemical provides customizable options including purity grades (e.g., 99%+ Pt basis), controlled hydration, and moisture-protected packaging to suit your synthesis, pharmaceutical, or research requirements.
Primary Applications of Potassium Tetrachloroplatinate(II)
Potassium Tetrachloroplatinate(II) serves as a fundamental Pt(II) synthon, enabling precise ligand exchange and complex formation in high-value chemical and pharmaceutical processes.
Cisplatin and Platinum Anticancer Agent Synthesis
Acts as the primary starting material in the industrial and laboratory preparation of cisplatin (cis-[PtCl₂(NH₃)₂]) and related Pt(II) therapeutics via controlled ammine substitution, ensuring high stereochemical purity.
Organometallic Pt(II) Complex Preparation
Facilitates synthesis of square-planar Pt(II) complexes with phosphines, amines, olefins, or other ligands for homogeneous catalysis, luminescent materials, and mechanistic studies.
Platinum Catalyst Precursor Development
Used in the generation of supported Pt catalysts or homogeneous Pt species through reduction or ligand modification for hydrogenation, hydrosilylation, and C-C coupling reactions.
Platinum Nanoparticle and Colloid Synthesis
Enables controlled reduction to produce Pt nanoparticles, clusters, or sols for electrocatalysis, sensors, and biomedical applications requiring defined particle size and high dispersion.
Coordination Chemistry Research
Serves as a standard Pt(II) source for exploring ligand substitution kinetics, trans effect, and new platinum-containing coordination compounds in academic and industrial labs.
Advanced Materials & Sensors
Supports preparation of Pt-modified surfaces, electrodes, or functional materials for gas sensors, biosensors, and electrochemical devices with enhanced performance.
Why Choose Aure Chemical for Your Potassium Tetrachloroplatinate(II) Supply?
Partner with Aure Chemical for Potassium Tetrachloroplatinate(II) to benefit from premium quality, technical expertise, and a secure precious metal supply chain for your platinum chemistry needs.
Consistent High Platinum Content
Strict analytical controls ensure accurate Pt assay, minimal impurities, and batch-to-batch reproducibility for dependable synthetic outcomes.
Secure Precious Metal Sourcing
Transparent, responsible platinum procurement with inventory management to provide supply stability and competitive pricing.
Technical Synthesis Guidance
Dedicated specialists offer advice on handling, ligand substitution protocols, reduction methods, and process optimization tailored to your applications.
Full Regulatory Compliance
Comprehensive documentation including COA, REACH, RoHS, and detailed SDS to streamline global operations and pharmaceutical-grade requirements.
Sustainability & Recycling Support
Ethical sourcing practices and platinum recovery/recycling programs aligned with ESG objectives and sustainable chemistry principles.
Hazards Classification
GHS Classification: Acute Toxicity, Oral (Category 3); Skin Corrosion (Category 1B); Serious Eye Damage (Category 1); Skin Sensitization (Category 1); Aquatic Chronic (Category 2)
Hazard Statements: H301: Toxic if swallowed; H314: Causes severe skin burns and eye damage; H317: May cause an allergic skin reaction; H411: Toxic to aquatic life with long lasting effects
UN Number: UN 3288
Hazard Class: 6.1 (Toxic substances)
Packing Group: III
 GHS06: Acute toxicity
GHS06: Acute toxicity GHS05: Corrosive
GHS05: Corrosive GHS07: Exclamation mark (sensitizer)
GHS07: Exclamation mark (sensitizer) GHS09: Environment (aquatic toxicity)
GHS09: Environment (aquatic toxicity)
