High-Purity Cisplatin from Aure Chemical – Your Trusted Source
Cisplatin, cis-diamminedichloroplatinum(II) or cis-[PtCl₂(NH₃)₂], CAS No. 15663-27-1, is a foundational platinum-based chemotherapeutic agent and one of the most widely used anticancer drugs in oncology. Supplied by Aure Chemical as a bright yellow to yellow-orange crystalline powder with high platinum content (approximately 64.8–65.0% Pt), this square-planar Pt(II) complex exhibits potent antitumor activity through DNA crosslinking and is a critical active pharmaceutical ingredient (API) in approved cancer treatments. Its clinical efficacy against testicular, ovarian, bladder, lung, and head/neck cancers, combined with well-characterized pharmacology, maintains its essential role in modern chemotherapy regimens. Aure Chemical provides pharmaceutical-grade material with stringent impurity control, batch-to-batch consistency, and full compliance documentation to support drug product manufacturing, clinical research, and formulation development worldwide.
Basic Information of Cisplatin
Aure Chemical supplies Cisplatin as a pharmaceutical-grade platinum(II) coordination complex with verified high purity and controlled platinum content for oncology API applications.
| CAS No. | 15663-27-1 |
|---|
| EC No. | 239-622-4 |
|---|
| Chemical Formula | Pt(NH3)2Cl2 |
|---|
| Molecular Weight | 300.05 g/mol |
|---|
| Appearance | Bright yellow to yellow-orange crystalline powder |
|---|
| Odor | Odorless |
|---|
| Melting point | Decomposes >270 °C without melting |
|---|
| Boiling point | Not applicable (decomposes) |
|---|
| Density | approx. 3.70 g/cm³ |
|---|
| Solubility | Sparingly soluble in water (~2.5 mg/mL at 25 °C); insoluble in most organic solvents |
|---|
| Nature (hazards) | Toxic if swallowed, causes severe skin burns and eye damage, may cause allergic skin reaction, suspected carcinogen, toxic to aquatic life with long lasting effects |
|---|
| RIDADR | UN 3288, Toxic solid, inorganic, n.o.s. (Cisplatin), Class 6.1, Packing Group II |
|---|
| Chemical Structure | 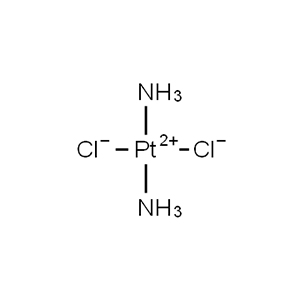 |
|---|
Aure Chemical offers customizable options including purity grades (≥99.5% typically), controlled impurity profiles (per ICH Q3A/Q3D), stability-indicating packaging, and batch sizes tailored to pharmaceutical manufacturing and clinical supply needs.
Primary Applications of Cisplatin
Cisplatin remains a cornerstone chemotherapeutic agent, delivering proven clinical efficacy across multiple cancer types through its unique DNA-binding mechanism.
Testicular Cancer Treatment
Serves as the standard first-line therapy for advanced testicular germ cell tumors, achieving high cure rates in combination regimens due to its exceptional sensitivity in these malignancies.
Ovarian Cancer Chemotherapy
Used in frontline and recurrent platinum-sensitive ovarian cancer protocols, often in combination with taxanes or other agents, providing significant progression-free and overall survival benefits.
Bladder and Urothelial Cancer
Forms the backbone of neoadjuvant, adjuvant, and metastatic cisplatin-based chemotherapy for muscle-invasive and advanced urothelial carcinoma.
Head and Neck Squamous Cell Carcinoma
Essential component of concurrent chemoradiotherapy and induction regimens for locally advanced head and neck cancers, improving locoregional control and survival.
Lung Cancer (NSCLC)
Included in platinum doublet regimens for advanced non-small cell lung cancer, particularly in combination with gemcitabine, pemetrexed, or vinorelbine for non-squamous histology.
Pharmaceutical Research & Combination Therapies
Employed in clinical trials, liposomal formulations, nanoparticle delivery systems, and combination studies to enhance efficacy and reduce toxicity in modern oncology development.
Why Choose Aure Chemical for Your Cisplatin Supply?
Trust Aure Chemical as your strategic partner for Cisplatin API, combining pharmaceutical-grade quality, regulatory expertise, and stable platinum supply for oncology manufacturing and research.
Pharmaceutical-Grade Purity & Compliance
Manufactured under stringent controls with full ICH-compliant impurity profiling, stability data, and Certificates of Analysis to meet global pharmacopeial standards (USP, EP, JP).
Precise Platinum Content Control
Accurate Pt assay and low residual metal impurities ensure batch consistency and optimal drug product performance.
Secure & Transparent Precious Metal Sourcing
Responsible platinum procurement with traceability and inventory buffering to mitigate supply risks and price volatility.
Regulatory & Technical Support
Comprehensive DMF support, stability studies, and expert guidance on formulation, handling, and process validation for seamless integration into drug product manufacturing.
Commitment to Oncology & Sustainability
Dedicated to supporting life-saving therapies with ethical sourcing, platinum recycling programs, and ESG-aligned practices for responsible pharmaceutical supply.
Hazards Classification
GHS Classification: Acute Toxicity, Oral (Category 2); Skin Corrosion (Category 1B); Serious Eye Damage (Category 1); Skin Sensitization (Category 1); Germ Cell Mutagenicity (Category 2); Carcinogenicity (Category 1B); Reproductive Toxicity (Category 1B); Specific Target Organ Toxicity, Repeated Exposure (Category 1) (kidney, bone marrow); Aquatic Chronic (Category 1)
Hazard Statements: H300: Fatal if swallowed; H314: Causes severe skin burns and eye damage; H317: May cause an allergic skin reaction; H341: Suspected of causing genetic defects; H350: May cause cancer; H360: May damage fertility or the unborn child; H372: Causes damage to organs through prolonged or repeated exposure; H410: Very toxic to aquatic life with long lasting effects
UN Number: UN 3288
Hazard Class: 6.1 (Toxic substances)
Packing Group: II
 GHS06: Acute toxicity
GHS06: Acute toxicity GHS05: Corrosive
GHS05: Corrosive GHS08: Health hazard (carcinogen, mutagen, reproductive toxicity)
GHS08: Health hazard (carcinogen, mutagen, reproductive toxicity) GHS09: Environment (aquatic toxicity)
GHS09: Environment (aquatic toxicity)
