Trimethylsilyl Chloride Structure and Chemical Properties Explained
Trimethylsilyl chloride (TMSCl) is an organosilicon compound and silyl halide with the chemical formula (CH₃)₃SiCl, commonly used as a reagent in organic synthesis. It is a colorless, volatile liquid that fumes in moist air due to its reactivity with water. Synonyms include chlorotrimethylsilane, trimethylchlorosilane, and TMCS. Its CAS number is 75-77-4. TMSCl plays a crucial role in organic synthesis and organosilicon chemistry, particularly as a silylating agent for protecting functional groups and enhancing volatility in analytical techniques like gas chromatography. This article provides an overview of its structure and properties, covering chemical identity, structure, physical and chemical properties, safety, and handling.
Chemical Identity and Nomenclature
The molecular formula of TMSCl is C₃H₉ClSi. Its molecular weight is 108.64 g/mol. The IUPAC name is chlorotrimethylsilane, though it is also referred to as chlorotri(methyl)silane in some contexts. Common trade names and abbreviations include TMSCl, chlorotrimethylsilane, and trimethylchlorosilane. Registry identifiers include CAS number 75-77-4, PubChem CID 6397, and EC number 200-900-5.
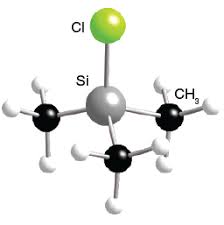 Structure of Trimethylsilyl Chloride
Structure of Trimethylsilyl Chloride
The structural formula of TMSCl features a central silicon atom bonded to three methyl groups (CH₃) and one chlorine atom. The Si–Cl bond serves as the primary reactive center due to its polarity and susceptibility to nucleophilic attack. The molecule adopts a tetrahedral geometry around the silicon atom, consistent with sp³ hybridization. Compared to other methylchlorosilanes, such as dichlorodimethylsilane ((CH₃)₂SiCl₂), which has two chlorine atoms and is more reactive toward hydrolysis, or tetramethylsilane ((CH₃)₄Si), which lacks a halogen and is inert to many reactions, TMSCl strikes a balance with a single reactive Si–Cl bond while maintaining stability in anhydrous conditions.
Physical Properties
TMSCl is a colorless liquid that fumes in moist air, producing hydrogen chloride upon contact with humidity. Its boiling point is 57 °C (135 °F), and its melting point is −40 °C (−40 °F). The density is approximately 0.856 g/cm³ at 25 °C. The refractive index is 1.387 at 20 °C. Vapor pressure is around 100 mm Hg at 25 °C. Regarding solubility, TMSCl reacts vigorously with water but is miscible with organic solvents such as ether, benzene, diethyl ether, and perchloroethylene.
Chemical Properties and Reactivity
TMSCl undergoes hydrolysis with water to produce trimethylsilanol ((CH₃)₃SiOH) and hydrochloric acid (HCl), though in practice, it often forms hexamethyldisiloxane ((CH₃)₃Si-O-Si(CH₃)₃) due to further condensation: 2 (CH₃)₃SiCl + H₂O → (CH₃)₃Si-O-Si(CH₃)₃ + 2 HCl. The polar Si–Cl bond imparts high reactivity, making it susceptible to nucleophiles. It is commonly employed as a silylating agent to protect alcohols, amines, and carboxylic acids by forming trimethylsilyl ethers, amines, or esters, which can be easily removed later. Under anhydrous conditions, TMSCl is stable, but it reacts violently with moisture, strong oxidizing agents, acids, bases, aldehydes, alcohols, amines, esters, and ketones. Stability concerns include potential decomposition in the presence of water, leading to HCl release, and it should be handled to avoid such risks.
Safety and Handling Overview
TMSCl poses several hazards: it is highly flammable (flash point −28 °C), corrosive, and toxic if swallowed, inhaled, or in contact with skin. Hydrolysis produces hazardous HCl gas, which can cause respiratory irritation and burns. Personal protective equipment (PPE) requirements include chemical-resistant gloves, safety goggles, face protection, and use in a well-ventilated fume hood. Storage recommendations involve keeping it in airtight containers under an inert atmosphere (e.g., nitrogen) in a cool, dry, well-ventilated place, away from ignition sources and incompatible materials. Regulatory notes include GHS classifications such as H225 (highly flammable liquid), H301+H311+H331 (toxic if swallowed, in contact with skin, or inhaled), H314 (causes severe skin burns and eye damage), and compliance with REACH, OSHA, and other standards.



The structure of TMSCl, centered on the reactive Si–Cl bond, underpins its versatile chemistry in silylation and protection strategies. Its physical properties as a low-boiling, dense liquid and chemical reactivity with nucleophiles make it indispensable in organic synthesis, despite handling challenges posed by its moisture sensitivity and flammability. For further reading, explore applications in protecting group chemistry, such as in "Protective Groups in Organic Synthesis" by Greene and Wuts, or detailed reviews on silylating agents in organosilicon literature.
Related Articles
How is Trimethylsilyl Chloride Produced? Industrial Routes and Methods
Dichlorodimethylsilane vs Trimethylchlorosilane: What's the Difference?
Applications of Trimethylsilyl Chloride in Pharmaceuticals and Biotechnology
Trimethylsilyl Chloride in Polymer Chemistry and Material Science
Protecting Groups in Organic Chemistry: The Role of Trimethylsilyl Chloride
Looking for a reliable bulk supplier of Trimethylsilyl Chloride?
Aure Chemical provides Premium Trimethylsilyl Chloride (TMSCl) raw materials.
View our Trimethylsilyl Chloride (TMSCl) product page
