Dichlorodimethylsilane vs Trimethylchlorosilane: What's the Difference?
Organosilicon compounds are a class of substances that feature carbon-silicon bonds, bridging organic and inorganic chemistry. These compounds are foundational in modern materials science, particularly in the production of silicones, which offer unique properties like thermal stability, flexibility, and water repellency. Dichlorodimethylsilane (CAS 75-78-5) and trimethylchlorosilane (CAS 75-77-4) are two prominent chlorosilanes—organosilicon compounds containing chlorine atoms bonded to silicon—that are often compared due to their roles as key intermediates in silicone synthesis and related chemical processes. Both arise as products or byproducts in industrial reactions like the direct synthesis of methylchlorosilanes, where they share similar production pathways but differ in functionality and applications.
The purpose of this article is to highlight the similarities and differences between dichlorodimethylsilane and trimethylchlorosilane, providing insights into their structures, properties, uses, and handling to aid researchers, manufacturers, and chemists in selecting the appropriate compound for specific needs.
Overview of Dichlorodimethylsilane (CAS 75-78-5)
Chemical Formula and Molecular Structure
Dichlorodimethylsilane has the chemical formula C₂H₆Cl₂Si, commonly written as (CH₃)₂SiCl₂. It features a tetrahedral molecular structure 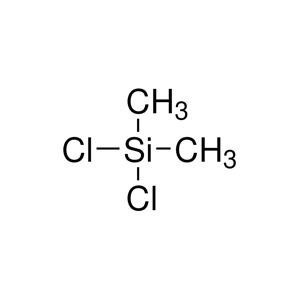 centered on a silicon atom, with two methyl groups (CH₃) and two chlorine atoms (Cl) bonded to it.
centered on a silicon atom, with two methyl groups (CH₃) and two chlorine atoms (Cl) bonded to it.
Physical Properties
This compound appears as a colorless liquid with a density of 1.07 g/cm³. It has a melting point of -76°C and a boiling point of 70°C, making it relatively volatile. Its flash point is -9°C, indicating high flammability.
Common Applications
Dichlorodimethylsilane is primarily used in the production of silicone polymers, resins, and elastomers, where it serves as a key monomer for forming Si-O chains through hydrolysis and condensation. It also acts as a coupling agent in specialty chemicals and is employed in the synthesis of polysilanes, which are precursors to silicon carbide. Additionally, it finds use in surface coatings, such as treating glass to prevent micro-particle adsorption.
Safety and Handling Considerations
Dichlorodimethylsilane is highly reactive with moisture, releasing hydrochloric acid (HCl) upon hydrolysis, which can cause severe irritation to skin, eyes, and respiratory systems. It is classified as a flammable liquid and irritant, requiring handling in a moisture-free environment with appropriate personal protective equipment (PPE) like gloves, goggles, and respirators. Avoid contact with water or air to prevent violent reactions.
Overview of Trimethylchlorosilane (CAS 75-77-4)
Chemical Formula and Molecular Structure
Trimethylchlorosilane has the chemical formula C₃H₉ClSi, often denoted as (CH₃)₃SiCl or TMSCl. Its molecular structure is also tetrahedral,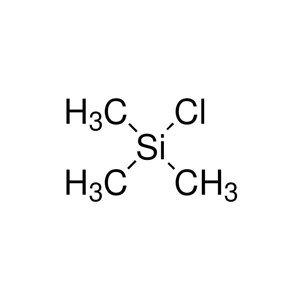 with the silicon atom bonded to three methyl groups and one chlorine atom.
with the silicon atom bonded to three methyl groups and one chlorine atom.
Physical Properties
It is a colorless liquid that fumes in moist air, with a density of 0.856 g/cm³. The melting point is -40°C, boiling point is 57°C, flash point is -28°C, and autoignition temperature is 400°C, highlighting its higher volatility compared to dichlorodimethylsilane.
Common Applications
Trimethylchlorosilane is widely used as a silylation reagent in organic synthesis, forming protecting groups for alcohols, amines, and other functional groups to facilitate reactions like gas chromatography or Mukaiyama aldol additions. It is employed in surface modification, such as silanizing laboratory glassware to make surfaces lipophilic, and in pharmaceuticals, coatings, and the production of other silyl derivatives. It also aids in dehydrating metal chlorides and generating anhydrous HCl solutions.
Safety and Handling Considerations
This compound is highly flammable, toxic if swallowed or inhaled, and causes severe skin burns and eye damage. It is suspected of causing cancer and reacts vigorously with water to release HCl. Handling requires explosion-proof equipment, moisture exclusion, and full PPE, with operations conducted in well-ventilated areas.
Key Similarities
Both dichlorodimethylsilane and trimethylchlorosilane are chlorosilanes, sharing a silicon-chlorine bond that makes them reactive intermediates in organosilicon chemistry. They are produced via similar industrial processes, such as the direct synthesis from methyl chloride and silicon, and both exhibit moisture sensitivity, hydrolyzing to release HCl and form siloxane structures. Their industrial relevance is evident in the silicone sector, valued at billions annually, where they contribute to materials with broad applications in electronics, construction, and consumer products.
Main Differences
Molecular Structure
Dichlorodimethylsilane features two methyl groups and two chlorine atoms ((CH₃)₂SiCl₂), while trimethylchlorosilane has three methyl groups and one chlorine atom ((CH₃)₃SiCl). This difference in substitution affects their functionality—dichlorodimethylsilane is difunctional, allowing for polymerization, whereas trimethylchlorosilane is monofunctional.
Reactivity
The higher chlorine substitution in dichlorodimethylsilane leads to greater reactivity, including faster hydrolysis rates and easier formation of extended chains or networks. Trimethylchlorosilane, with one chlorine, hydrolyzes to form simpler dimers like hexamethyldisiloxane and is less prone to polymerization.
Applications
Dichlorodimethylsilane is mainly used for producing silicone elastomers and resins through crosslinking, while trimethylchlorosilane excels in end-capping polymer chains, surface treatments, and as a protecting agent in synthesis.
Dichlorodimethylsilane:mainly a starting material for silicones (bulk industrial use).
Silicone polymer production: It is a key monomer/intermediate in the Müller–Rochow process, forming the backbone for silicones such as polydimethylsiloxane (PDMS).
Resins and elastomers: Used in the production of silicone resins, rubbers, and fluids with applications in construction, electronics, and coatings.
Intermediate for organosilicon compounds: Acts as a building block for a wide range of specialty chemicals, including siloxanes and silazanes.
Coupling and surface-modification agents: In some cases, modified derivatives improve adhesion or alter surface properties.
Trimethylchlorosilane → mainly a reactive reagent for end-capping, surface treatment, and silylation (specialty/fine chemical use).
Silylation reagent: Widely used in organic synthesis and analytical chemistry to protect functional groups (e.g., converting alcohols or acids into trimethylsilyl derivatives).
End-capping agent in silicone chemistry: Reacts with silanol groups to terminate silicone polymer chains, controlling molecular weight and properties.
Surface treatment: Applied to glass, silica, or metal oxides to render surfaces hydrophobic (water-repellent).
Pharmaceuticals and fine chemicals: Acts as a reagent in drug synthesis and advanced intermediates.
Coatings and electronics: Used in preparing water- and chemical-resistant coatings, and in some semiconductor processes.
Physical Properties
Trimethylchlorosilane is more volatile (boiling point 57°C vs. 70°C) and less dense (0.856 g/cm³ vs. 1.07 g/cm³), influencing handling and separation in industrial processes.
| Property | Dichlorodimethylsilane | Trimethylchlorosilane |
| Molecular Formula | C₂H₆Cl₂Si | C₃H₉ClSi |
| Molecular Weight | 129.06 g/mol | 108.64 g/mol |
| Appearance | Colorless fuming liquid with a pungent odor | Colorless fuming liquid with a pungent odor |
| Density | 1.0745 g/cm³ (at 20°C) | 0.858 g/cm³ (at 25°C) |
| Melting Point | -76°C | -57.7°C |
| Boiling Point | 70°C | 57.6°C |
| Flash Point | -8.9°C (16°F) | -27°C |
| Refractive Index | 1.3998 (at 20°C) | 1.3885 (at 20°C) |
| Vapor Density (air = 1) | 4.4 | 3.75 (calculated) |
| Solubility in Water | Insoluble | Reacts vigorously |
Industrial Implications
The structural differences—difunctional vs. monofunctional—directly dictate their uses: dichlorodimethylsilane's two reactive sites enable it to form polymeric networks in silicones, ideal for elastomers and resins, while trimethylchlorosilane's single site suits chain termination or precise modifications. Manufacturers might choose dichlorodimethylsilane for bulk silicone production in industries like automotive, construction, and electronics, where durable materials are needed. In contrast, trimethylchlorosilane is preferred for research, pharmaceuticals, and coatings requiring silylation or surface hydrophobization. Typical industries for dichlorodimethylsilane include silicone manufacturing and advanced materials, while trimethylchlorosilane supports optoelectronics, microelectronics, and chemical synthesis.
Safety and Storage Considerations
Shared Hazards
Both compounds are corrosive, flammable, and release HCl upon contact with moisture, posing risks of skin burns, eye damage, and respiratory irritation. They are also toxic and require careful management to avoid fires or violent reactions.
Recommended Storage Conditions
Store in tightly closed containers under inert gas (e.g., nitrogen) in a cool, dry, well-ventilated area away from heat, sparks, and moisture. Use grounded containers to prevent static buildup, and segregate from incompatible materials like water or bases.
Key Handling Precautions
Handle in fume hoods with explosion-proof equipment, wearing chemical-resistant PPE. Avoid skin/eye contact, inhalation, and ingestion. In case of spills, use vapor-suppressing foam and neutralize with appropriate agents. Follow good industrial hygiene practices, including washing after handling.
In summary, dichlorodimethylsilane and trimethylchlorosilane differ primarily in structure (difunctional vs. monofunctional), reactivity (higher for dichlorodimethylsilane), applications (polymer production vs. silylation and surface treatment), and physical properties (e.g., boiling point and density). Select dichlorodimethylsilane for applications requiring crosslinking in silicones, and trimethylchlorosilane for protective or terminal modifications in synthesis or coatings. Regardless of choice, prioritize regulatory compliance and safety protocols, as both pose significant hazards if mishandled, emphasizing the need for proper training and equipment in any working environment.

