How is Trimethylsilyl Chloride Produced? Industrial Routes and Methods
Trimethylsilyl chloride ((CH₃)₃SiCl), also known as chlorotrimethylsilane, is a cornerstone organosilicon compound. As a volatile, colorless liquid, its importance in modern chemistry cannot be overstated. It serves as a vital silylating agent for protecting functional groups in complex organic synthesis—especially in the pharmaceutical industry—and as a chain-terminating or "end-capping" agent in the production of silicone polymers. This article details the industrial routes and methods used to manufacture this indispensable chemical reagent.
Overview of Trimethylsilyl Chloride Production
Industrially, trimethylsilyl chloride (TMSCl) is not typically produced as a standalone product. Instead, it is one component of a mixture of methylchlorosilanes generated through a process developed independently by Eugene G. Rochow and Richard Müller in the 1940s. This landmark discovery, now known as the Müller–Rochow Process or the Direct Process, remains the global standard for producing the precursors to silicone materials. In this process, TMSCl is a valuable co-product, with the primary target usually being dimethyldichlorosilane ((CH₃)₂SiCl₂), the main monomer for silicone polymers.
The Direct Process (Müller–Rochow Process)
The Direct Process is a gas-solid reaction that forms carbon-silicon bonds on a massive scale. It is the most economically viable route for all methylchlorosilanes.
The fundamental reaction involves passing gaseous methyl chloride (CH₃Cl) over heated, powdered elemental silicon (Si) in the presence of a catalyst. The reaction yields a mixture of products:
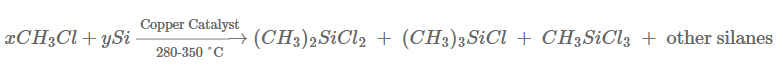
The reaction is highly exothermic and is carried out in a fluidized bed reactor to ensure uniform temperature and efficient contact between the gas and solid reactants. The typical product distribution by weight is:
Dimethyldichlorosilane ((CH₃)₂SiCl₂): 70–90%
Methyltrichlorosilane (CH₃SiCl₃): 5–15%
Trimethylsilyl chloride ((CH₃)₃SiCl): 2–5%
After the reaction, the crude mixture of hot gases is cooled and condensed. The liquid methylchlorosilanes are then separated into high-purity fractions using a series of large-scale fractional distillation columns, which exploit the differences in their boiling points.
Laboratory and Alternative Synthetic Routes

While the Direct Process dominates industrially, other methods exist for synthesizing TMSCl, primarily for laboratory-scale usewhere cost is less of a concern.
From Hexamethyldisilane: The reaction of hexamethyldisilane ((CH₃)₃Si–Si(CH₃)₃) with gaseous chlorine (Cl₂) or hydrogen chloride (HCl) can produce TMSCl. This route is not economical for bulk production due to the high cost of the starting disilane.
From Hexamethyldisiloxane: Hexamethyldisiloxane (((CH₃)₃Si)₂O), a common byproduct of silicone chemistry, can be cleaved using various chlorinating agents. A common lab preparation involves reacting it with thionyl chloride (SOCl₂) and a catalyst like ferric chloride (FeCl₃).
These routes, while effective, cannot compete with the efficiency and scalability of the Müller–Rochow Process and are therefore not used for industrial manufacturing.
Process Conditions and Yield Considerations
Optimizing the Direct Process is critical for maximizing the output of desired products. Key factors include:
Catalysts: Copper is the essential catalyst, typically used in the form of copper(I) chloride (CuCl) or as a mixture of copper oxides. Promoters such as zinc, tin, or antimony are often added to the catalyst system to improve reaction rates and selectivity towards specific silanes.
Temperature and Pressure: The reaction is highly sensitive to temperature, which is tightly controlled between 280–350 °C. The pressure is typically kept slightly above atmospheric.
Reactant Purity and Ratio: High-purity silicon (>98%) and methyl chloride are required. Adjusting the CH₃Cl/Si feed ratio can slightly influence the product distribution.
Purification: Achieving the high purity (>99%) required for pharmaceutical and electronic applications demands highly efficient multi-stage distillation to remove closely boiling isomers and other trace impurities.
Industrial Relevance and Supply Chain
TMSCl holds a unique position in the chemical industry. Its production is intrinsically linked to the multi-billion dollar silicone market. Large-scale chemical manufacturers produce methylchlorosilanes primarily to make silicone fluids, elastomers, and resins. TMSCl is isolated as a valuable side stream from this main production line. Its role as a potent silylating agent makes it indispensable for creating volatile derivatives for gas chromatography and for introducing protecting groups during the synthesis of active pharmaceutical ingredients (APIs).
Safety and Environmental Considerations
The production of TMSCl involves significant hazards that require stringent controls.
Hazards: Methyl chloride is a flammable and toxic gas. Chlorosilanes, including TMSCl, are corrosive and react vigorously with water and moisture in the air to release flammable hydrogen gas and corrosive hydrochloric acid (HCl).
Handling: The entire process must be conducted in sealed, closed systems to prevent the release of hazardous materials. Personnel must use specialized personal protective equipment (PPE).
Environmental Impact: Modern plants focus on green chemistry principles by improving catalyst efficiency to reduce waste, recycling unreacted methyl chloride, and converting unwanted byproducts into more useful materials.
Conclusion
The industrial production of trimethylsilyl chloride is a story of efficiency and co-dependence. It relies almost exclusively on the Müller–Rochow (Direct) Process, a cornerstone of organosilicon chemistry. Although it is a minor component of the initial product mixture, TMSCl is meticulously separated via fractional distillation to become a high-value reagent. Its availability as a co-product from the massive silicone industry enables critical applications across organic synthesis, materials science, and analytics, cementing its role as a small-volume but high-impact chemical workhorse.
Related Articles
Trimethylsilyl Chloride Structure and Chemical Properties Explained
Dichlorodimethylsilane vs Trimethylchlorosilane: What's the Difference?
Applications of Trimethylsilyl Chloride in Pharmaceuticals and Biotechnology
Trimethylsilyl Chloride in Polymer Chemistry and Material Science
Protecting Groups in Organic Chemistry: The Role of Trimethylsilyl Chloride
Looking for a reliable bulk supplier of Trimethylsilyl Chloride?
Aure Chemical provides Premium Trimethylsilyl Chloride (TMSCl) raw materials.
View our Trimethylsilyl Chloride (TMSCl) product page
