Safe Handling and Storage Guidelines for Cyclohexane
Introduction
Cyclohexane, with the chemical formula C₆H₁₂, is a cycloalkane classified as a non-polar, colorless, flammable liquid exhibiting a distinctive sweet, gasoline-like or detergent-like odor. It is immiscible in water but soluble in organic solvents such as ether, alcohol, and acetone. Industrially, cyclohexane is primarily produced through the hydrogenation of benzene and serves as a key precursor in the manufacture of adipic acid and caprolactam, which are essential for nylon production. Additional uses include functioning as a solvent in chemical processes, paint and varnish removers, oil extractants, lacquers, resins, fats, oils, waxes, solid fuels, and even as a substitute for benzene in various applications. Proper handling and storage of cyclohexane are critical due to its high flammability, which poses risks of fire and explosion, as well as its health hazards including skin irritation, central nervous system effects, and aspiration risks if ingested. Environmentally, it is very toxic to aquatic life with long-lasting effects, necessitating strict controls to prevent releases that could harm ecosystems.
Chemical Profile of Cyclohexane
Chemical Formula & Structure
Cyclohexane has the molecular formula C₆H₁₂ and consists of a six-carbon cyclic structure. The molecule adopts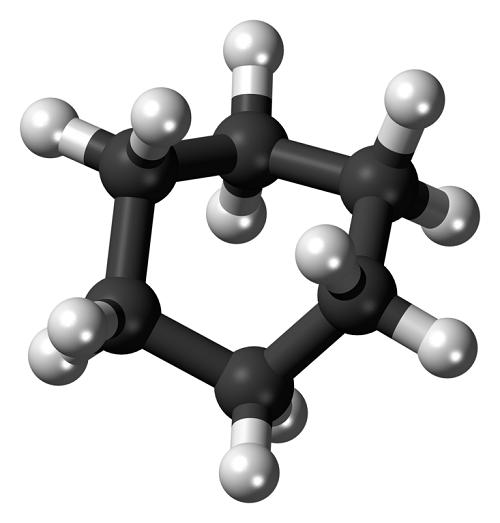 a three-dimensional chair conformation to minimize torsional strain, with carbon atoms positioned at a tetrahedral angle of 109.5°. In this stable form, half of the hydrogen atoms are equatorial (lying in the plane of the ring), while the other half are axial (perpendicular to the plane).
a three-dimensional chair conformation to minimize torsional strain, with carbon atoms positioned at a tetrahedral angle of 109.5°. In this stable form, half of the hydrogen atoms are equatorial (lying in the plane of the ring), while the other half are axial (perpendicular to the plane).
Physical Properties
Cyclohexane exhibits several key physical properties that influence its handling and storage requirements. The following table summarizes these characteristics based on reliable data:
Property | Value/Description |
Appearance | Colorlessliquid |
Odor | Sweet, pungent, gasoline-like |
Boiling Point | 80.74 °C (177°F) |
Melting Point | 6.47 °C (44 °F) |
Flash Point | -20 °C (-4 °F) |
Solubility | Insoluble in water; soluble in ether, alcohol, acetone |
Vapor Pressure | 78 mmHg at 20 °C (or 95 mmHg at 68 °F) |
Specific Gravity | 0.8 (water = 1) |
Vapor Density | 2.9 (air = 1) |
Auto Ignition Temperature | 473 °F (245 °C) |
Molecular Weight | 84.2 |
Hazard Classification
Under the Globally Harmonized System (GHS) and OSHA standards, cyclohexane is classified as a flammable liquid (Category 2), skin irritant (Category 2), specific target organ toxicity - single exposure (Category 3, central nervous system), aspiration hazard (Category 1), and aquatic hazard (acute and chronic Category 1). Hazard statements include H225 (highly flammable liquid and vapor), H304 (may be fatal if swallowed and enters airways), H315 (causes skin irritation), H336 (may cause drowsiness or dizziness), and H410 (very toxic to aquatic life with long-lasting effects). It is also listed by agencies such as OSHA, ACGIH, DOT, NIOSH, DEP, NFPA, and EPA.
Health & Safety Hazards
Health Risks
Cyclohexane poses health risks through inhalation, skin or eye contact, and ingestion. Inhalation can irritate the nose and throat, leading to coughing and wheezing, while skin or eye contact may cause irritation and burns. Ingestion carries a high risk of aspiration, which can be fatal if the substance enters the airways.
Acute vs. Chronic Effects
Acute exposure may result in headache, dizziness, nausea, vomiting, lightheadedness, drowsiness, and loss of consciousness. High concentrations can be lethal, with documented LD50 values of 12,705 mg/kg (oral, rat) and 813 mg/kg (oral, mouse). Chronic effects include skin rash, dryness, itching, redness, and potential damage to the liver and kidneys. Combined with alcohol consumption, it may exacerbate liver damage.
Flammability Risks
As a highly flammable substance, cyclohexane vapors can form explosive mixtures with air (explosive limits: 1.3%–8.4%), with risks heightened by static electricity ignition or flow/agitation generating electrostatic charges. Vapors are heavier than air and may travel to distant ignition sources, causing flashbacks or explosions in confined spaces.
Personal Protective Equipment (PPE)
To mitigate risks, appropriate PPE is essential. Chemical-resistant gloves should be worn to prevent skin contact and irritation. Eye protection, such as safety goggles or a face shield, is required to guard against splashes that could cause burns. Protective clothing, including lab coats or coveralls, helps minimize skin exposure. Respiratory protection, such as a NIOSH-approved full-facepiece respirator with an organic vapor cartridge, is necessary when ventilation is insufficient, particularly for exposures exceeding 100 ppm. Exposure limits include TWA 100 ppm (ACGIH), 300 ppm (NIOSH REL/OSHA Z-1).
Safe Handling Guidelines
Cyclohexane should always be used in well-ventilated areas or under fume hoods to prevent vapor accumulation. Avoid sparks, flames, and static discharge by grounding and bonding containers during transfers. Proper labeling of containers and use of secondary containment are recommended to contain potential leaks. Safe transfer techniques include using spark-proof tools, explosion-proof equipment, and working under an inert gas when necessary. Do not inhale vapors or aerosols, and ensure no smoking in handling areas.
Storage Requirements
Storage Conditions
Store cyclohexane in a cool, dry, well-ventilated area, ideally at temperatures between 15–25 °C, to maintain stability. Keep containers tightly closed and upright in designated, labeled areas such as chemical storage cabinets.
Temperature & Ventilation Considerations
Protect from sunlight and heat sources, ensuring adequate local and general ventilation to prevent vapor buildup. Use approved flammable-liquid containers, such as mild steel drums.
Segregation from Incompatible Chemicals
Store away from oxidizers (e.g., perchlorates, peroxides, chlorine), acids, nitrates, and other incompatible substances like nitrogen dioxide, as it reacts explosively with them. Secondary containment, such as polypropylene tubs, is advised.
Emergency Measures
Spill Response
For spills, evacuate the area, ensure ventilation, and avoid ignition sources. Cover drains, absorb with materials like Chemizorb® or other liquid-absorbents, and dispose properly without creating vapors. Use spark-proof tools and explosion-proof equipment.
Fire Response
Use foam, dry chemical, CO₂, or alcohol-resistant foam extinguishers; water may be ineffective but can cool containers. Firefighters should wear self-contained breathing apparatus and keep a safe distance.
First Aid Measures
Inhalation: Move to fresh air; if breathing stops, perform rescue breathing or CPR, and seek medical attention.
Skin/Eye Contact: Remove contaminated clothing; wash skin with soap and water; flush eyes with water for 15 minutes, removing contact lenses.
Ingestion: Do not induce vomiting; immediately call a poison center or doctor due to aspiration risk.
Environmental Considerations
Cyclohexane should not be released into soil, waterways, or drains, as it is very toxic to aquatic life and can cause long-lasting effects. Prevent entry into surface or groundwater during firefighting or spills. Disposal must follow hazardous waste regulations, routing contents and containers to approved facilities.
Regulatory Compliance
Compliance with standards such as OSHA (e.g., Respiratory Protection Standard 29 CFR 1910.134), NFPA, EU CLP, and GHS is required. Exposure limits include PEL/REL of 300 ppm (1,050 mg/m³) and IDLH of 1,300 ppm. For transportation, it is classified under UN number 1145, hazard class 3 (flammable liquids).
Strict adherence to these handling and storage guidelines is essential to minimize risks associated with cyclohexane's flammability, health effects, and environmental impact. Fostering a strong workplace safety culture, including proper training and use of PPE, significantly reduces potential hazards and promotes overall risk minimization.
Looking for a reliable bulk supplier of Cyclohexane?
Aure Chemical provides Premium Cyclohexane raw materials.
View our Cyclohexane product page
