How is Ethylidene Chloride (CAS 75-34-3) Produced? Industrial Routes and Laboratory Methods
Ethylidene chloride, also known as 1,1-dichloroethane (CAS 75-34-3), is a chlorinated hydrocarbon with the molecular formula C₂H₄Cl₂ or CH₃CHCl₂. As a volatile organic compound, it serves as an important chemical intermediate in the synthesis of other substances, including 1,1,1-trichloroethane, vinyl chloride, and various solvents, adhesives, and synthetic fibers. This article explores its industrial production routes, laboratory synthesis methods, process considerations, and safety aspects, based on the provided outline and supplemented with reliable sources.
Industrial Production Overview
Ethylidene chloride is typically produced as a secondary or co-product in chlorination processes rather than as a primary target compound. It belongs to the family of chlorinated ethanes and is manufactured in large volumes, with annual U.S. production exceeding 1 million pounds. Its production often occurs alongside related compounds like 1,2-dichloroethane, with isolation depending on downstream applications in chemical synthesis.
Industrial Routes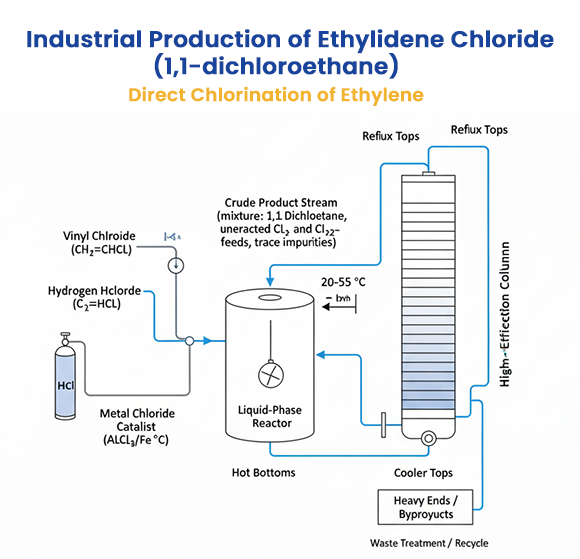
Direct Chlorination of Ethylene
One common industrial route involves the direct chlorination of ethylene, which can yield a mixture of 1,1-dichloroethane and1,2-dichloroethane under controlled conditions. The reaction proceeds via addition of chlorine (Cl₂) to ethylene (C₂H₄), but product distribution favors 1,2-dichloroethane unless modified by catalysts or conditions to enhance 1,1-isomer formation. However, a more specific and efficient method for 1,1-dichloroethane is the catalytic addition of hydrogen chloride (HCl) to vinyl chloride (CH₂=CHCl), typically at 20–55 °C in the presence of metal chloride catalysts like aluminum chloride (AlCl₃), ferric chloride (FeCl₃), or zinc chloride (ZnCl₂):
CH₂=CHCl + HCl → CH₃CHCl₂
This process achieves high selectivity and is the primary commercial method.
Catalytic or Thermal Chlorination of Ethane
Another route is the catalytic or thermal chlorination of ethane (C₂H₆), where stepwise substitution of hydrogen atoms with chlorine leads to various chloroethanes, including 1,1-dichloroethane as a minor product. The reaction is conducted at elevated temperatures (300–500 °C) or photochemically, with chlorine gas:
C₂H₆ + Cl₂ → C₂H₅Cl + HCl
C₂H₅Cl + Cl₂ → CH₃CHCl₂ (1,1-isomer) or CH₂ClCH₂Cl (1,2-isomer) + HCl
Product ratios depend on temperature, chlorine-to-ethane ratio, and catalysts (e.g., metal halides). While this method produces 1,1-dichloroethane in smaller proportions, it is integrated into broader chlorinated hydrocarbon production. Additionally, 1,1-dichloroethane can form as a by-product during vinyl chloride production via cracking of 1,2-dichloroethane or in chloral manufacture.
Separation and Purification
The crude mixture from chlorination processes is separated via fractional distillation, exploiting differences in boiling points: 1,1-dichloroethane (57.2 °C) vs. 1,2-dichloroethane (83.5 °C). High-efficiency distillation columns are used to isolate the compound, often followed by washing or adsorption to remove impurities like HCl or unreacted chlorine. Industrial-grade purity is typically 95–99%, while laboratory-grade requires further refinement to >99.5% for analytical uses.
Laboratory Synthesis Methods
Laboratory methods focus on small-scale, controlled reactions for research or educational purposes, emphasizing selectivity over yield.
Controlled Chlorination of Ethane/Ethylene
Similar to industrial routes but on a smaller scale, chlorination of ethane or ethylene can be performed photochemically (UV light) or thermally in glassware. For example, bubbling chlorine into ethane at low temperatures (0–50 °C) with light initiation yields 1,1-dichloroethane, though mixtures require distillation. Conditions include pressure control (1–5 atm) to favor the 1,1-isomer. Addition of HCl to vinyl chloride in a solvent like dichloromethane, catalyzed by AlCl₃, is also adaptable for lab use.
Organic Synthetic Routes
A classic laboratory method involves the reaction of acetaldehyde (CH₃CHO) with phosphorus pentachloride (PCl₅) to form the geminal dihalide:
CH₃CHO + PCl₅ → CH₃CHCl₂ + POCl₃ + HCl
This substitution reaction is conducted in anhydrous conditions and is useful for teaching organic halogenation. Alternative routes include further chlorination of ethyl chloride (CH₃CH₂Cl) using Cl₂ under UV light or catalytic conditions. Grignard reagents are less common but could involve ethylmagnesium chloride with chlorinating agents, though this is atypical for dihalides.
Process Considerations
Selectivity challenges arise in controlling the ratio of 1,1- vs. 1,2-dichloroethane, influenced by catalyst type, temperature, and reactant ratios—lower temperatures and specific catalysts favor the 1,1-isomer. Safety aspects include handling hazardous chlorine gas and corrosive HCl by-products, requiring sealed systems and ventilation. Economically, production is tied to demand for co-products like 1,2-dichloroethane, with plants optimizing for vinyl chloride feedstock; 1,1-dichloroethane isolation adds value as a solvent or intermediate.
Safety, Handling, and Environmental Aspects
Ethylidene chloride is flammable (flash point -17 °C), toxic by inhalation (causing CNS effects, dizziness, and potential carcinogenicity), and a skin irritant. In production, handle in well-ventilated areas with PPE, avoiding ignition sources and incompatibles like strong oxidizers. Environmental releases occur during manufacturing, processing, and disposal, contributing to air and water pollution; it is volatile (vapor pressure 182 mmHg at 20 °C) with a half-life of ~62 days in the atmosphere. Regulatory compliance includes EPA risk evaluations, waste management via incineration or recycling, and minimizing emissions to prevent groundwater contamination.
Ethylidene chloride is primarily produced industrially through the addition of HCl to vinyl chloride or as a co-product in ethylene/ethane chlorination processes. Laboratory methods, such as chlorination or reactions with PCl₅, offer alternatives for small-scale needs but are inefficient for bulk production. Its availability relies on integrated chlorinated ethane manufacturing, with emphasis on selectivity, safety, and environmental controls. For quality and safety, source from reputable suppliers specializing in industrial chemicals.
Related Articles
Looking for a reliable bulk supplier of Ethylidene Chloride (CAS 75-34-3)?
Aure Chemical provides Premium Ethylidene Chloride (CAS 75-34-3) raw materials.
View our Ethylidene Chloride (CAS 75-34-3) product page
