Ethylidene Chloride: Structure, Physical Properties, and Chemical Behavior
Ethylidene chloride, also known as 1,1-dichloroethane (CAS 75-34-3), is a chlorinated hydrocarbon widely used in organic chemistry as a solvent and intermediate in chemical synthesis. Its relevance stems from applications in producing other chlorinated compounds, such as 1,1,1-trichloroethane, and its role in thermal cracking to vinyl chloride, a key monomer for polymers. This article covers its molecular structure, physical properties, chemical behavior including reactivity and combustion, comparisons with related compounds, and safety considerations, drawing from the provided outline and supplemented with verified sources.
Molecular Structure
Ethylidene chloride has the molecular formula C₂H₄Cl₂ and a molar mass of 98.96 g/mol. It is structurally represented as CH₃-CHCl₂,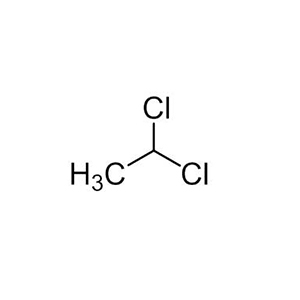
where both chlorine atoms are attached to the same carbon atom (the alpha carbon), making it a geminal dihalide. This distinguishes it from its isomer, 1,2-dichloroethane (CH₂Cl-CH₂Cl), which is a vicinal dihalide. In line notation, it can be depicted as C-C(Cl)(Cl), with the first carbon having three hydrogens and the second having one hydrogen. The ball-and-stick model shows a tetrahedral geometry around the chlorinated carbon, with C-Cl bonds approximately 1.77 Å and C-C bond about 1.52 Å. There is no stereoisomerism due to the lack of chiral centers, but it exhibits constitutional isomerism with 1,2-dichloroethane, leading to differing chemical behaviors.
Physical Properties
Ethylidene chloride appears as a clear, colorless liquid with a sweet, chloroform-like odor. It is denser than water and exhibits low solubility in aqueous media but mixes well with organic solvents. Key physical constants are summarized in the table below for quick reference:
| Property | Value | Notes/Source |
| Appearance | Colorless oily liquid | |
Odor | Chloroform-like | |
Density | 1.2 g/cm³ (at 20°C) | |
Melting Point | -97 °C (-143 °F) | |
Boiling Point | 57.2 °C (135 °F) | |
Solubility in Water | 0.6% (at 20°C) | Low; volatile in water |
Solubility in Organics | Miscible | With alcohols, ethers, etc. |
Vapor Pressure | 182 mmHg (at 20°C) | High volatility |
Flash Point | -17 °C (2 °F) | Highly flammable |
Explosive Limits | 5.4–11.4% | In air |
These properties make it suitable for use as a solvent in extractions and degreasing operations.
Chemical Properties
Reactivity
Ethylidene chloride is relatively stable under normal conditions but susceptible to dehydrohalogenation in the presence of strong bases, forming vinyl chloride (CH₂=CHCl) via elimination of HCl. This reaction occurs thermally at 400–500 °C under pressure or with bases like alcoholic KOH. It decomposes in the atmosphere with a half-life of about 62 days, primarily via reaction with hydroxyl radicals. As a geminal dihalide, it can undergo hydrolysis to acetaldehyde under certain conditions, differing from vicinal dihalides.
Combustion & Thermal Behavior
It is flammable with a low flash point, burning to produce carbon monoxide (CO), hydrogen chloride (HCl), and traces of phosgene (COCl₂) under incomplete combustion. Vapors are heavier than air and can travel to ignition sources, posing flashback risks.
Compatibility with Other Chemicals
Ethylidene chloride reacts with strong oxidizers, potentially leading to fire or explosion, and with alkali metals, forming reactive intermediates. It shows limited substitution reactivity compared to 1,2-dichloroethane due to the geminal structure, which favors elimination over nucleophilic attacks. It is moderately reactive among halogenated aliphatics, with reactivity decreasing as more hydrogens are replaced by halogens.
Comparison with Related Compounds
Ethylidene chloride (1,1-dichloroethane) and ethylene dichloride (1,2-dichloroethane) are isomers with similar molecular weights and chemical properties but differ in structure: geminal vs. vicinal halogen placement. This leads to distinct behaviors—1,1-dichloroethane hydrolyzes to acetaldehyde with aqueous alkali, while 1,2-dichloroethane forms ethane-1,2-diol. Reactivity-wise, 1,1 is used as a feedstock for 1,1,1-trichloroethane and cracks to vinyl chloride, whereas 1,2 is primarily for vinyl chloride production and other solvents. Physical properties are comparable (both colorless liquids), but applications diverge: 1,1 for degreasing and fumigants, 1,2 for broader solvent uses.
Ethyleidne Chloride (1,1-dichloroethane, CAS 75-34-3) belongs to the family of chlorinated ethanes. Closely related compounds include 1,2-dichloroethane (ethylene dichloride) and chloroethane. Although these compounds share the same molecular formula in some cases, structural differences lead to distinct physical properties, chemical behavior, and applications.
| Property / Feature | Ethylidene Chloride (1,1-DCE) | Ethylene Dichloride (1,2-DCE) |
| Molecular Formula | C₂H₄Cl₂ | C₂H₄Cl₂ |
| Structure | Both Cl on the same carbon | Cl on different carbons |
| Boiling Point | ≈ 57 °C | ≈ 83 °C |
| Main Use | Intermediate, specialty solvents | VCM production → PVC plastics |
| Stability / Reactivity | Prone to dehydrohalogenation | More stable; bulk industrial use |
Ethylidene Chloride and Ethylene Dichloride are structural isomers with distinct roles. While Ethylidene Chloride is less commercially dominant, it is important in specialty synthesis. Ethylene Dichloride is the key global feedstock for vinyl chloride and PVC. Understanding these differences helps researchers, manufacturers, and procurement teams select the right compound for industrial and laboratory applications.
Other Related Compounds
Chloroethane (C₂H₅Cl): single chlorine substitution; low boiling point; solvent and historical anesthetic.
Trichloroethanes (C₂H₃Cl₃): more halogenated; different solvent and toxicity profiles.
Safety and Environmental Considerations
Ethylidene chloride is toxic by inhalation, causing central nervous system effects like dizziness, nausea, and unconsciousness at moderate concentrations. It is a known carcinogen (California Proposition 65 listed since 1990) with a PEL of 100 ppm (TWA) and IDLH of 3000 ppm. Environmentally, it is volatile and persistent in air (half-life 62 days), contributing to atmospheric degradation. Handle in well-ventilated areas with PPE, avoiding skin contact (may cause burns) and ignition sources. Store in cool, dry places away from incompatibles like oxidizers. Spills should be contained with non-combustible absorbents and prevented from entering waterways.
Ethylidene chloride (1,1-dichloroethane) features a geminal dihalide structure (C₂H₄Cl₂, CH₃CHCl₂) with physical properties like a boiling point of 57.2°C and low water solubility, making it volatile and solvent-like. Chemically, it undergoes dehydrohalogenation to vinyl chloride, combusts to toxic gases, and shows compatibility issues with strong reagents. Compared to 1,2-dichloroethane, its structural difference drives unique reactivity and applications in organic synthesis and industry. While significant as an industrial chemical, its toxicity and carcinogenicity necessitate careful handling. For further reading, explore its industrial applications in synthesis and solvent uses.
Related Articles
Safety, Storage, and Handling Guidelines for Ethylidene Chloride (CAS 75-34-3)
Ethylidene Chloride vs. Ethylene Dichloride: What’s the Difference?
Applications of Ethylidene Chloride in Industry and Organic Synthesis
How is Ethylidene Chloride (CAS 75-34-3) Produced? Industrial Routes and Laboratory Methods
Looking for a reliable bulk supplier of Ethylidene Chloride (CAS 75-34-3)?
Aure Chemical provides Premium Ethylidene Chloride (CAS 75-34-3) raw materials.
View our Ethylidene Chloride (CAS 75-34-3) product page
