How to Prepare Hexachloroacetone via the Catalytic Liquid-phase Chlorination Reaction of Acetone
The primary method for producing hexachloroacetone involves a stepwise chlorination reaction where acetone or its chlorinated intermediates react with chlorine molecules (Cl₂) in a solvent, typically in the presence of a catalyst. The process can be summarized as follows:
Reaction Principle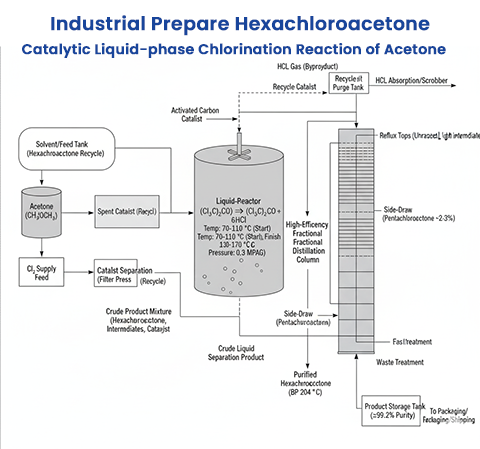 The reaction progressively substitutes hydrogen atoms in acetone with chlorine, ultimately forming hexachloroacetone:
The reaction progressively substitutes hydrogen atoms in acetone with chlorine, ultimately forming hexachloroacetone:
CH₃COCH₃ + Cl₂ → (Cl₃C)₂CO + HCl
This is a multi-step process, as acetone is chlorinated to form intermediates like monochloroacetone (CH₃COCH₂Cl), dichloroacetone, trichloroacetone, and so on, until all six hydrogens are replaced to yield hexachloroacetone. The general reaction for each step involves:
R₁R₂CO + Cl₂ → R₁R₂CCl₂O + HCl
Where R₁ and R₂ are progressively chlorinated methyl groups (e.g., CH₃, CH₂Cl, CHCl₂, CCl₃).
Key Process Details
Catalyst: Activated carbon is commonly used as a catalyst due to its ability to facilitate chlorination by adsorbing both acetone (or chloroacetones) and chlorine molecules, promoting the reaction on its surface. The mechanism involves:
Adsorption of acetone and chlorine onto activated carbon.
Chlorination reaction between adsorbed species.
Desorption of the chlorinated product (e.g., hexachloroacetone). Alternatively, catalysts like pyridine (0.05–5% by weight) or methyl pyridines (e.g., picoline) are used to enhance selectivity and reduce side reactions, such as the formation of mesityl oxide (a condensation product of acetone and HCl).
Solvent: The reaction is conducted in a liquid phase, often using hexachloroacetone itself as the solvent to minimize side reactions and maintain a homogeneous reaction medium. Other organohalogen compounds (C₁–C₃, with at least three halogen atoms, such as chloroform or carbon tetrachloride) may also be used.
Reaction Conditions:
Temperature: The process typically starts at lower temperatures (70–110 °C) for initial chlorination to di- or trichloroacetone, then increases to 130–170 °C for exhaustive chlorination to hexachloroacetone. Stepwise temperature increases prevent excessive side reactions.
Pressure: Reactions are often conducted at atmospheric pressure or slightly elevated (e.g., 0.3 MPaG) to control chlorine flow and reaction kinetics.
Molar Ratio: A high chlorine-to-acetone molar ratio (e.g., 7.7:1 or higher) ensures complete chlorination. The molar ratio of chlorine to hydrogen atoms in the starting material is typically ≥0.83 to achieve high yields.
Process Steps:
Reaction Step: Acetone or chloroacetones (monochloroacetone to pentachloroacetone) are reacted with chlorine in the presence of activated carbon in a solvent. The reaction mixture contains hexachloroacetone, intermediates, and the catalyst.
Catalyst Separation: Activated carbon is separated from the reaction mixture (e.g., by filtration) to obtain a crude product. The catalyst can be recovered and reused without significant loss of activity.
Purification: Fractional distillation isolates hexachloroacetone (boiling point ~204 °C) from impurities like pentachloroacetone (2–3% by mass) or other chloroacetones. Final purity can reach 97–99.8%, with commercial grades available at ≥99.2% or ≥99.8%.
Yield and Efficiency: Using activated carbon, yields of hexachloroacetone can reach 98.4% based on acetone, with minimal impurities (e.g., 0.5% trichloroacetone, 0.7% unreacted acetone). Pyridine-catalyzed processes yield ~90% based on dichloroacetone intermediates.
Advantages of the Process
High Yield: Activated carbon catalysis minimizes side reactions, achieving near-quantitative conversion.
Catalyst Recovery: Activated carbon is easily separated and reused, reducing costs.
Environmental Benefit: Utilizes waste streams (e.g., monochloroacetone or polychlorinated acetone by-products) from other processes, reducing industrial waste.
Challenges
Side Reactions: Without proper catalysis, hydrogen chloride (HCl) by-product can react with acetone to form mesityl oxide (CH₃C(=CH₂)COCH₃) or other condensation products, lowering yields. Catalysts like pyridine or controlled conditions mitigate this.
Safety: Chlorine gas and HCl are corrosive and toxic, requiring sealed systems and robust ventilation.
Selectivity: Achieving exhaustive chlorination without over-chlorination or incomplete reactions requires precise control of temperature and chlorine feed.
Laboratory Synthesis Methods
In laboratory settings, hexachloroacetone can be synthesized similarly but on a smaller scale, often for research or specialized applications:
Chlorination of Acetone: Conducted in a glass-lined reactor with chlorine gas bubbled through acetone containing a catalyst (e.g., 0.28% pyridine). The reaction starts at reflux (~56 °C for acetone), with temperatures gradually increased to 90–170 °C over hours. A molar ratio of acetone to chlorine of ~1:2 is maintained initially, with excess chlorine (10–20%) to ensure complete chlorination.
Use of Intermediates: Partially chlorinated acetones (e.g., 1,1-dichloroacetone) can be further chlorinated in the liquid phase at 110–170 °C with additional catalyst to yield hexachloroacetone.
Purification: Lab-scale distillation or recrystallization ensures high purity, though yields may be lower (80–90%) due to less optimized conditions compared to industrial processes.
Recent Advances
Recent developments, as noted in patent literature, emphasize the use of activated carbon in liquid-phase chlorination for high yields (up to 98.4%) and low impurity content. For example, a 2016 patent describes a process using hexachloroacetone as the solvent, with acetone fed at 0.023 mol/min and chlorine at 4 L/min at 150 °C and 0.3 MPaG, achieving a reaction mixture of 97.2% hexachloroacetone. Additionally, the process supports environmental sustainability by repurposing polychlorinated acetone waste from monochloroacetone production, converting it into hexachloroacetone for applications like deuterated chloroform synthesis.
Applications
Hexachloroacetone is used as a trichloroacetylating agent (similar to trichloroacetyl chloride), a pesticide, and a precursor for deuterated chloroform (CDCl₃) via hydrolysis with deuterium oxide and catalysts like pyridine or sodium deuteroxide. It also serves as a chlorinating agent in organic synthesis, such as for aryl chlorides or phosphines, and in the Perkow reaction for insect repellents.
Safety and Environmental Considerations
Toxicity: Hexachloroacetone is a colorless to yellowish liquid with a pungent odor, slightly soluble in water, and toxic upon inhalation or skin contact. It requires handling in well-ventilated areas with PPE.
Storage: Store in tightly closed containers in a cool, dry, well-ventilated place to maintain stability.
Environmental Impact: The process generates HCl, which must be neutralized or captured to prevent emissions. Using waste chloroacetones as feedstocks reduces environmental burden.
Conclusion
Hexachloroacetone is efficiently produced via catalytic liquid-phase chlorination of acetone or chloroacetones, using activated carbon or pyridine as catalysts and hexachloroacetone as a solvent. Industrial processes achieve high yields (up to 98.4%) with precise control of temperature (130–170 °C), chlorine feed, and purification via distillation. Laboratory methods mirror these but are less optimized. Recent advances focus on sustainability by utilizing waste streams, making the process economically and environmentally viable. For high-purity hexachloroacetone, sourcing from suppliers like Wacker Chemie AG ensures quality for industrial or research needs.
Looking for a reliable bulk supplier of Hexachloroacetone (CAS 116-16-5)?
Aure Chemical provides Premium Hexachloroacetone (CAS 116-16-5) raw materials.
View our Hexachloroacetone (CAS 116-16-5) product page
