Preparation of Hexachloroacetone (HCA, CAS 116-16-5) — Comprehensive Technical Guide
A detailed, engineering-oriented summary of HCA production: reaction principles, laboratory and industrial chlorination methods, catalyst choices, purification, analytics, plant safety and environmental control strategies.
Introduction & Key Properties
Hexachloroacetone (HCA, also called hexachloropropanone or perchloroacetone, CAS 116-16-5) has the molecular formula C₃Cl₆O and a molecular weight of approximately 264.75 g·mol⁻¹. It is a dense, slightly water-soluble chlorinated ketone that typically appears as a colorless to pale yellow liquid; reported density is ~1.74 g·mL⁻¹ and melting point near −2 to −3 °C. HCA is corrosive, produces toxic fumes on decomposition, and is handled as a hazardous/chlorinated intermediate in specialty chemical manufacture.
Reaction Chemistry & Mechanistic Overview
The industrially practical route to HCA is deep electrophilic chlorination of acetone (or stepwise chlorination of partially chlorinated acetones) whereby hydrogen atoms on the methyl groups adjacent to the carbonyl are replaced stepwise by chlorine until the fully substituted hexachloro product is formed. Mechanistically, the overall transformation proceeds via radical or electrophilic chlorination pathways (chain propagation via Cl under thermal or photochemical conditions, or ionic/electrophilic pathways when catalysts or Lewis acids are present). Side reactions can include oxidative cleavage, formation of lower-chlorinated by-products (mono- to pentachloroacetone), and hydrolysis products (e.g., trichloroacetic derivatives) if moisture is present.
Key notes: radical (UV/thermal) and catalyzed liquid-phase chlorination are commonly used; process control of temperature, Cl₂ feed rate, and catalyst selection dictates selectivity toward HCA.
Common Preparation Routes of Hexachloroacetone (HCA, CAS 116-16-5)
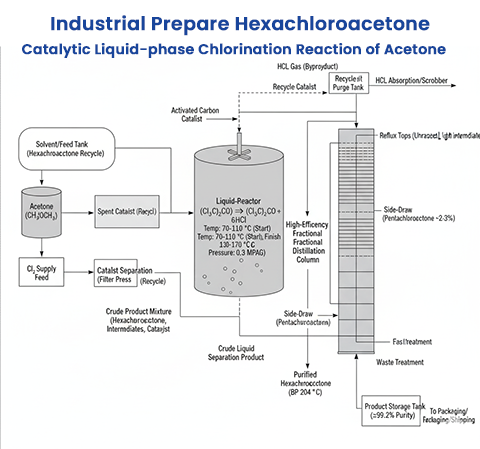
Hexachloroacetone (HCA) is an important chlorinated ketone used as an intermediate in specialty chemical synthesis and research applications. Its preparation has been studied through several routes, of which catalytic liquid-phase chlorination remains the mainstream industrial method. Below we describe several specific preparation methods in more detail.
Catalytic Liquid-Phase Chlorination of Acetone (Mainstream Industrial Route)
Reaction Principle: Acetone is chlorinated with chlorine gas under anhydrous conditions. A solid catalyst such as activated carbon, ferric chloride (FeCl₃), or carbon-supported catalysts promotes substitution of all six hydrogen atoms by chlorine.Conditions: Conducted in liquid phase with temperature control to avoid decomposition. Good mixing and optimized catalyst loading improve reaction rates.
Advantages: Simple feedstock (acetone + Cl₂), modest equipment, easy downstream purification by washing and vacuum distillation.
Industrial Practice: Patents describe recoverable catalysts that improve selectivity, reduce tar formation, and allow regeneration.
Photochemical (UV) Chlorination
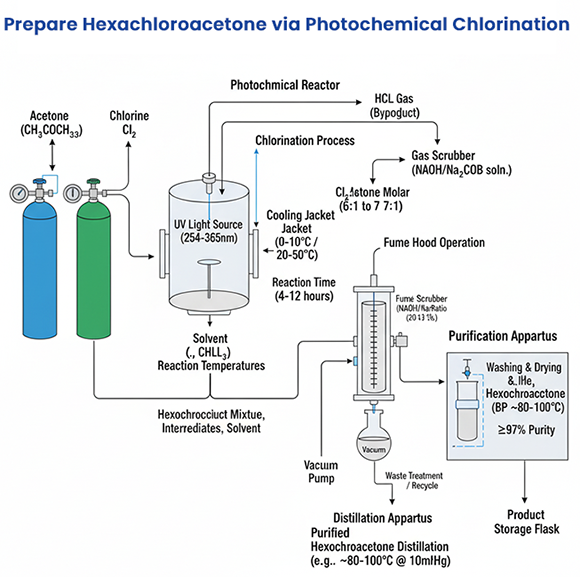
Reaction Principle: Chlorine gas is irradiated with UV light, generating radicals that attack acetone to form HCA.
Tuning: Photon flux, wavelength, and residence time can be adjusted to promote deeper chlorination.
Drawbacks: Expensive UV reactors, high operating costs, safety risks with UV and radicals, by-product formation from overheating.
Use Cases: Specialized laboratory or niche industrial settings.
Chlorinating Reagents (PCl₅, SOCl₂, etc.) — Less Common
Examples: Phosphorus pentachloride, thionyl chloride, or oxalyl chloride can chlorinate acetone or partially chlorinated acetones.
Limitations: High cost, corrosive by-products, environmental disposal issues.
Practical Use: Laboratory scale for related perchlorinated ketones; not suitable for industrial production.
Alternative / Specialty Routes
Stepwise Chlorination: Further chlorination of trichloroacetone or pentachloroacetone to yield HCA.
Precursor Methods: Isopropanol derivatives oxidized and chlorinated to give HCA.
Patent Innovations: Solvent-assisted chlorination or novel catalyst systems to improve selectivity, reduce tar, and simplify catalyst recovery.
Among the available methods, catalytic liquid-phase chlorination of acetone remains the mainstream industrial route for HCA, combining cost-effectiveness and scalability. Photochemical chlorination provides high reaction rates but at greater expense and risk, while reagent-based and specialty methods are mostly confined to laboratory or patent-driven contexts.
Laboratory-Scale Batch Procedure (Detailed)
The following is a representative laboratory batch method intended for technical understanding only (not a protocol to be executed without institutional approvals and PPE). Quantities and vessel sizes should be scaled and engineered for safety when moving to pilot or production.
Equipment & Materials
Use a corrosion-resistant, closed reaction vessel (glass or enamel-lined), mechanical stirrer, external temperature control (jacketed bath), Cl₂ gas cylinder with regulator and gas-inlet sparger or sintered glass diffuser, gas scrubbing train (NaOH and Na₂S₂O₃ tanks), condensers and cold traps, and instrumentation for temperature, pressure, and off-gas monitoring. Start materials: anhydrous acetone (≥99.5%), dry chlorine (Cl₂) gas, activated carbon or FeCl₃ catalyst (pre-treated), and inert drying agents for workup.
Typical Stepwise Procedure
Charge acetone to the reactor to ≤ 30–40% of vessel volume to allow gas sparging and headspace. Add activated carbon catalyst (typically 1–10 wt% relative to acetone) or a small amount milligram-scale FeCl₃ for lab trials.
Establish an inert purge (N₂) and confirm the system is dry. Start agitation (200–800 rpm depending on vessel geometry).
Heat to controlled reaction temperature (commonly 35–55 °C for liquid-phase catalyzed routes). Maintain ≤60 °C to avoid runaway and decomposition.
Initiate slow Cl₂ sparge. Carefully control Cl₂ molar feed rate to match consumption; typical initial bubble frequency is low (few bubbles per second) and increased gradually. Monitor reaction exotherm; use cooling to keep temperature within target band.
Periodically sample reaction mixture; analytical methods (GC, density, or titration) track chlorination progress. As chlorination proceeds, viscosity and density rise; when near complete conversion to pentachloro/hexachloro species, slow Cl₂ to avoid over-chlorination side reactions and high local concentrations.
Upon reaching target conversion/selectivity (lab endpoints often determined by density or GC profiles), stop Cl₂ feed, purge with N₂, cool and transfer reaction mixture to separation/wash station.
Work-up & Purification (Lab)
Separate catalyst (filtration). Wash organic phase with saturated NaCl solution to remove inorganic acids, then with dilute NaHCO₃ if acidic impurities present. Dry over anhydrous MgSO₄ or Na₂SO₄, filter, and perform reduced-pressure distillation. Typical lab distillation: collect fraction in the characteristic boiling range (note that reported boiling points depend strongly on pressure). Analytical checks (GC, NMR) confirm HCA identity and purity.
Industrial/Continuous Process & Reactor Design
For scale-up, batch chlorination has limitations (heat removal, gas–liquid mass transfer, operator exposure). Continuous liquid-phase chlorination in tubular or loop reactors offers better heat management, narrower residence time distributions and improved selectivity. Modern designs use segmented cooling zones, online Cl₂ metering tied to inline GC or density sensors, and catalyst beds that can be regenerated or replaced. Patents describe activated carbon fixed-bed or slurry reactors that allow catalyst separation & regeneration to reduce impurity formation and improve overall yield.
Key Engineering Controls
Heat removal: Jacketed reactor, internal coil, or multi-zone tubular reactors to control exotherm.
Gas–liquid contact: Efficient spargers or static mixers to enhance Cl₂ dissolution and limit gas-phase pockets.
Inline monitoring: Density, refractive index or GC sampling to determine endpoint and avoid over-chlorination.
Tail-gas treatment: Multi-stage scrubbing (NaOH → Na₂S₂O₃) and oxidizing scrubbers for HCl/Cl₂ removal.

Purification, Analytical Methods & Quality Control
HCA must be characterized and tested for halogenation completeness, residual chlorinated by-products, water content and acid residues. Typical QC suite:
Analytical Methods
Gas Chromatography (GC): Main tool for quantifying chlorinated species and determining purity; use non-polar column (e.g., HP-5 or equivalent) with suitable temperature program. Reported retention times vary by method; validate method with standards.
NMR (¹H, ¹³C): Confirm full substitution pattern (no remaining methyl protons) and detect structural impurities.
Density & Refractive Index: Rapid checks to detect conversion stage (density increases with chlorination degree).
Halide/Titration: Silver nitrate test for free chlorides (after hydrolysis) to check completeness of substitution; careful sample prep required to avoid false positives.
Elemental Analysis / HRMS: Confirm molecular weight and chlorine content when high-precision verification is needed.
Packing & Storage
Store HCA in corrosion-resistant containers (HDPE or lined steel) in cool, ventilated areas, away from strong bases and reducing agents. Labeling per GHS and local transport regulations is mandatory. Small-scale samples should be kept in amber bottles under inert atmosphere when long term storage is required. Product samples for distribution typically specify percent purity (≥98% for many technical uses) and water content limits.
Safety, Emissions & Waste Treatment
HCA and its chlorination process produce highly hazardous streams (Cl₂, HCl, chlorinated organics). All operations must be designed for containment, remote operation where possible, and robust emergency scrubbing. Safety Data Sheets and industrial supplier guidance highlight acute toxicity and corrosivity—personnel exposure must be minimized and PPE rigorously enforced.
Tail-gas & Liquid Waste Management
Tail gases containing residual Cl₂ and HCl are commonly treated in a two-stage scrubber: primary alkaline scrub (NaOH) to neutralize HCl and remove Cl₂ by formation of chloride; followed by a reducing scrubber (sodium thiosulfate, Na₂S₂O₃) to quench remaining oxidants. Implement continuous monitoring of scrubber pH, Cl₂ and HCl concentrations and provide redundancy for critical scrubber pumps. Liquid wastes containing chlorinated organics often require advanced oxidation (Fenton) or thermal destruction, and must be disposed of per hazardous waste regulations. Demonstrated industrial solutions reach >99% Cl₂ removal when engineered correctly.
Personal Protection & Plant Controls
Full chemical-resistant suits, face shields, and self-contained breathing apparatus (SCBA) for emergency intervention.
Continuous ambient and personal monitoring for chlorine, HCl, and other VOCs in areas with potential leaks.
Explosion-proof electrical systems and grounding to avoid static sparks in gas handling zones.
Troubleshooting, Optimization & Common Failure Modes
Common issues include low yield, dark or polymeric product, and excessive acidic impurities. Typical causes and remedies:
Local overheating / charring: Improve agitation and cooling; reduce Cl₂ feed spikes.
Low conversion: Check Cl₂ purity, remove moisture, verify catalyst activity.
High acid content (HCl/trichloroacetic derivatives): Ensure effective salt washes and control hydrolysis sources (avoid water ingress).
Catalyst deactivation: For activated carbon catalysts, thermal regeneration (e.g., 500 °C in inert atmosphere) may restore activity; track regeneration cycles to avoid loss of performance.
Process improvements often focus on enhanced mass transfer (static mixers, microreactors), distributed cooling (multi-zone tubular reactors) and process analytical technology (PAT) for inline endpoint detection to avoid over-chlorination and by-product formation.
Looking for a reliable bulk supplier of Hexachloroacetone (CAS 116-16-5)?
Aure Chemical provides Premium Hexachloroacetone (CAS 116-16-5) raw materials.
View our Hexachloroacetone (CAS 116-16-5) product page
