How to via Photochemical Chlorination to Prepare Hexachloroacetone
Preparing hexachloroacetone ((Cl₃C)₂CO, CAS 116-16-5) via photochemical (UV) chlorination of acetone or its partially chlorinated derivatives is a viable laboratory method, though it is less common industrially due to the preference for catalytic liquid-phase chlorination. Below is a detailed explanation of how to synthesize hexachloroacetone using photochemical chlorination, based on chemical principles and available information.
Overview of Photochemical Chlorination
Photochemical chlorination involves the use of ultraviolet (UV) light to initiate a free radical chain reaction between acetone (CH₃COCH₃) or chloroacetone intermediates and chlorine gas (Cl₂). UV light provides the energy to break the Cl–Cl bond, generating chlorine radicals that substitute hydrogen atoms on the acetone molecule, progressively forming hexachloroacetone. The reaction is:
CH₃COCH₃ + 6 Cl₂ → (Cl₃C)₂CO + 6 HCl
This is a stepwise process, producing intermediates like monochloroacetone (CH₃COCH₂Cl), dichloroacetone, and so forth, until all six hydrogens are replaced.
Laboratory Synthesis Procedure
Materials and Equipment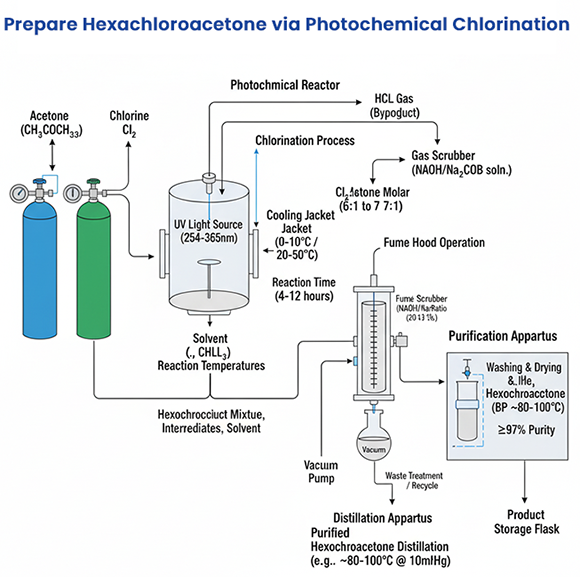
Starting Material: Acetone (CH₃COCH₃, reagent grade) or partially chlorinated acetones (e.g., 1,1-dichloroacetone).
Chlorine Source: Chlorine gas (Cl₂, high purity, from a cylinder with a regulator).
Solvent: Hexachloroacetone or a chlorinated solvent like chloroform (CHCl₃) or carbon tetrachloride (CCl₄) to maintain a liquid phase and reduce side reactions.
UV Light Source: A medium-pressure mercury vapor lamp (100–400 W, emitting at 254–365 nm) or equivalent UV source.
Reactor: A UV-transparent quartz or borosilicate glass reactor (e.g., a photochemical reactor with a cooling jacket).
Safety Equipment: Fume hood, personal protective equipment (PPE) including gloves, goggles, and respiratory protection, due to the toxicity of chlorine and hexachloroacetone.
Gas Trap: A scrubber with aqueous sodium hydroxide (NaOH) or sodium carbonate (Na₂CO₃) to neutralize HCl by-product.
Distillation Apparatus: For purification of the final product.
Reaction Setup and Conditions
Setup the Reactor:
Assemble the photochemical reactor in a fume hood. Ensure the quartz or glass reaction vessel is clean and dry.
Place the UV lamp inside a protective quartz sleeve or position it externally to irradiate the reaction mixture.
Connect a chlorine gas cylinder to the reactor via a gas inlet with a flow regulator. Attach an outlet to a scrubber to capture HCl gas.
Prepare the Reaction Mixture:
Add 50 mL of acetone (0.86 mol) to the reactor. Optionally, use a chlorinated solvent (e.g., 50–100 mL chloroform) to dilute the acetone and enhance solubility of chlorine.
Alternatively, start with a partially chlorinated acetone (e.g., 1,1-dichloroacetone) to reduce reaction time, though pure acetone is more common in lab settings.
Chlorination Process:
Cool the reactor to 0–10 °C using a cooling jacket to control the exothermic reaction and minimize side reactions like condensation to mesityl oxide (CH₃C(=CH₂)COCH₃).
Begin bubbling chlorine gas slowly into the reaction mixture (flow rate ~0.1–0.5 L/min, adjusted to avoid excessive pressure buildup).
Activate the UV lamp to initiate the reaction. UV light (254–365 nm) cleaves Cl₂ into Cl• radicals, which attack the methyl groups of acetone:
CH₃COCH₃ + Cl• → CH₃COCH₂• + HCl
CH₃COCH₂• + Cl₂ → CH₃COCH₂Cl + Cl•
Continue chlorination, monitoring the reaction progress by sampling and analyzing via gas chromatography (GC) or NMR for the formation of intermediates (e.g., CH₃COCH₂Cl, CH₃COCHCl₂, up to (Cl₃C)₂CO).
Reaction Conditions:
Temperature: Start at 0–10 °C for initial chlorination to monochloroacetone or dichloroacetone, then gradually increase to 20–50 °C as chlorination progresses to avoid decomposition or side products. Higher temperatures (e.g., 70–100 °C) may be needed for exhaustive chlorination to hexachloroacetone.
Chlorine Molar Ratio: Use a chlorine-to-acetone molar ratio of ~6:1 to 7:1 to ensure complete substitution (6 hydrogens replaced). This translates to ~4.8–5.6 mol Cl₂ per 0.86 mol acetone.
Reaction Time: Typically 4–12 hours, depending on UV intensity, chlorine flow rate, and degree of chlorination. Monitor until hexachloroacetone dominates the product mixture.
Pressure: Maintain near atmospheric pressure (1–1.5 atm) to control gas flow and prevent reactor overpressure.
Termination and Workup:
Stop chlorine flow and UV irradiation when analysis confirms high conversion to hexachloroacetone (typically >90% by GC).
Purge the reactor with nitrogen to remove residual chlorine gas, directing exhaust to the scrubber.
Transfer the reaction mixture to a separatory funnel or flask for further processing.
Purification
Distillation: Purify the crude product by fractional distillation under reduced pressure (e.g., 10–20 mmHg) to avoid decomposition. Hexachloroacetone has a boiling point of ~204 °C at 760 mmHg, but lower under vacuum (~80–100 °C at 10 mmHg). Collect the fraction containing hexachloroacetone (purity >97%).
Washing: If impurities like HCl or unreacted chlorine remain, wash the crude product with aqueous sodium bicarbonate (NaHCO₃) and dry over anhydrous sodium sulfate (Na₂SO₄).
Verification: Confirm purity via GC-MS or ¹H/¹³C NMR. Hexachloroacetone shows no ¹H NMR signals (no hydrogens) and a characteristic ¹³C carbonyl peak at ~180 ppm and CCl₃ peaks at ~80–90 ppm.
Yield
Yields in photochemical chlorination are typically 70–90% in lab settings, lower than industrial catalytic methods (up to 98%) due to side reactions and less optimized conditions. Starting with partially chlorinated acetones (e.g., trichloroacetone) can improve yields but requires prior synthesis.
Safety and Handling
Chlorine Gas: Highly toxic and corrosive. Work in a well-ventilated fume hood with a chlorine detector. Use a scrubber to neutralize HCl and unreacted Cl₂.
Hexachloroacetone: Toxic by inhalation and skin contact, with a pungent odor. Wear nitrile gloves, safety goggles, and a lab coat. Avoid prolonged exposure.
UV Light: UV radiation is harmful to eyes and skin. Use UV-blocking goggles and ensure the lamp is shielded.
Flammability: Acetone and early chloroacetone intermediates are flammable. Keep ignition sources away and ensure the reactor is sealed.
Storage: Store hexachloroacetone in a tightly sealed, dark glass container in a cool, dry, well-ventilated area to prevent decomposition or moisture absorption.
Challenges and Considerations
Selectivity: Photochemical chlorination can produce a mixture of chloroacetones (mono- to pentachloro-). Careful control of chlorine feed and UV exposure minimizes over- or under-chlorination.
Side Reactions: HCl by-product can catalyze acetone condensation to mesityl oxide, reducing yield. Low temperatures and a solvent like chloroform mitigate this.
Scale Limitations: Photochemical methods are less efficient for large-scale production due to UV penetration limits and energy costs compared to catalytic liquid-phase processes.
Environmental Impact: Neutralize HCl waste with NaOH or Na₂CO₃ and dispose of chlorinated waste per local regulations to avoid environmental contamination.
Alternative Notes
While photochemical chlorination is effective in the lab, industrial production prefers catalytic liquid-phase chlorination with activated carbon or pyridine for higher yields and better control (as discussed in prior responses). If starting from a partially chlorinated acetone, photochemical methods can complement catalytic steps by finishing the chlorination under UV light.
Conclusion
Hexachloroacetone can be synthesized in the laboratory via photochemical (UV) chlorination of acetone by bubbling chlorine gas through a cooled acetone solution under UV irradiation (254–365 nm), followed by fractional distillation to isolate the product. The process requires careful control of temperature (0–50 °C), chlorine flow, and reaction time (4–12 hours) to achieve yields of 70–90%. Safety precautions are critical due to the toxicity of chlorine and hexachloroacetone. For higher efficiency, consider catalytic methods, but photochemical chlorination is valuable for small-scale research or educational purposes.
Looking for a reliable bulk supplier of Hexachloroacetone (CAS 116-16-5)?
Aure Chemical provides Premium Hexachloroacetone (CAS 116-16-5) raw materials.
View our Hexachloroacetone (CAS 116-16-5) product page
