How is Dichloromethylsilane (CAS 75-54-7) Produced? Industrial Routes and Laboratory Methods
Dichloromethylsilane, also known as methyldichlorosilane, has the chemical formula CH₃SiHCl₂ (note: the provided document lists it as CH₂Cl₂SiH₂, which appears to be a typographical error; the correct formula is CH₃SiHCl₂). It is a key organosilicon compound used as a building block in the synthesis of silanes, silicones, and polymers, particularly for hydrosilylation reactions and the production of specialty silicon-based materials. This overview examines its industrial production and laboratory synthesis methods, drawing from the outlined structure and supplemented with verified online sources.
Overview of Industrial Production
Dichloromethylsilane is primarily obtained as a by-product rather than a targeted primary product in large-scale organosilicon manufacturing. The main industrial route is the Müller-Rochow process (also called the Direct Process), where it forms in minor quantities during the production of methylchlorosilanes from methyl chloride and silicon. In this process, the dominant product is dimethyldichlorosilane (70–90% yield), but dichloromethylsilane accounts for 1–4% of the product mixture, alongside other compounds like methyltrichlorosilane (5–15%) and trimethylsilyl chloride (2–4%). This by-product status makes its isolation economically viable only within the context of broader methylchlorosilane streams.
The Müller–Rochow (Direct) Process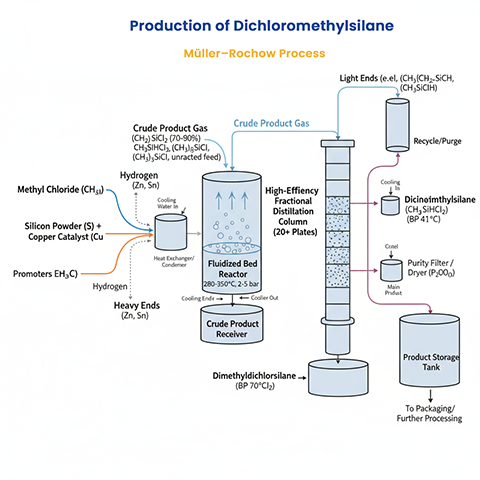
Reaction Principle
The Müller–Rochow process is a copper-catalyzed gas–solid reaction between methyl chloride (CH₃Cl) and elemental silicon (Si) at elevated temperatures. The general reaction is:
x CH₃Cl + Si → (CH₃)ₙSiCl₄₋ₙ + other products (where n = 1–3)
Specifically, the primary reaction aims for dimethyldichlorosilane:
2 CH₃Cl + Si → (CH₃)₂SiCl₂
However, a mixture of products forms, including dichloromethylsilane (CH₃SiHCl₂) as a minor hydrido compound. The presence of hydrogen or side reactions introduces Si–H bonds, leading to its formation. Conditions include temperatures of 280–350°C (typically 300°C), pressures of 2–5 bar, and a fluidized bed reactor with crushed silicon. Copper (often as Cu₃Si intermetallic) serves as the catalyst, enhanced by promoters like zinc, tin, and trace metals (e.g., aluminum, antimony) to improve selectivity and yield. Conversion rates are high: 90–98% for silicon and 30–90% for methyl chloride. Adding hydrogen to the gas phase can increase the selectivity for dichloromethylsilane and similar hydrosilanes to over 80 mol%.
Product Separation
The crude product mixture is separated via fractional distillation using high-efficiency columns (e.g., with 20+ theoretical plates) due to close boiling points: dichloromethylsilane (41°C), dimethylchlorosilane (35°C), trimethylsilyl chloride (57°C), methyltrichlorosilane (66°C), and dimethyldichlorosilane (70°C). Dichloromethylsilane is isolated from lower-boiling fractions. Purity is critical, with electronic-grade requiring minimal impurities; it is often stored over P₂O₅ to protect from moisture.
Laboratory Synthesis Methods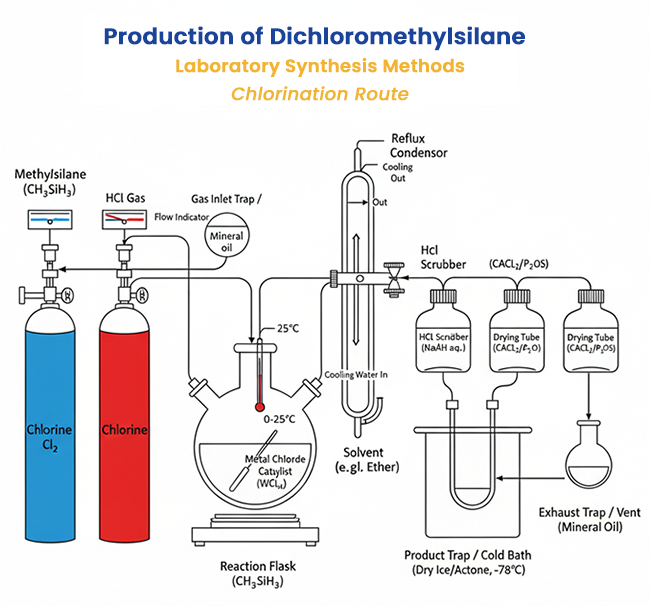
Laboratory methods are typically small-scale and focus on controlled reactions, though they are less cost-effective for industrial volumes. They include chlorination routes and Grignard-based alternatives.
Chlorination Routes
Controlled chlorination of methylsilane (CH₃SiH₃) or related hydrosilanes uses chlorine (Cl₂), hydrogen chloride (HCl), or other chlorinating agents to replace H with Cl stepwise. For example:
CH₃SiH₃ + Cl₂ → CH₃SiH₂Cl + HCl
CH₃SiH₂Cl + Cl₂ → CH₃SiHCl₂ + HCl
The reaction occurs in the presence of HCl to enhance chlorination, often catalyzed by metal chlorides (e.g., WCl₆, MoCl₅). Conditions are managed to avoid over-chlorination to methyltrichlorosilane (CH₃SiCl₃). This method is suitable for lab-scale due to its selectivity under mild conditions.
Another approach involves high-temperature hydrogenation/reduction of methyltrichlorosilane:
CH₃SiCl₃ + H₂ → CH₃SiHCl₂ + HCl
This partial reduction can achieve 30% conversion under CVD-like conditions or with catalysts, though it's more common in industrial contexts for yield enhancement.
Alternative Routes
Grignard reactions provide a versatile lab method. Methylmagnesium chloride (CH₃MgCl) reacts with trichlorosilane (HSiCl₃) to substitute one Cl with CH₃:
CH₃MgCl + HSiCl₃ → CH₃SiHCl₂ + MgCl₂
This occurs in anhydrous ether or THF solvents, with controlled addition to prevent further substitution. The Grignard approach is classic for forming Si–C bonds and is adaptable for small-scale synthesis of organosilanes. Silicon tetrachloride (SiCl₄) can also be used stepwise, but requires additional reduction steps for the Si–H bond.
Process Considerations
Selectivity in the Müller–Rochow process depends on catalyst composition, temperature, and CH₃Cl/Si ratio; promoters like zinc reduce coke formation and enhance yields. By-product management involves recycling unwanted fractions or converting them (e.g., disproportionation). Purity varies: industrial-grade tolerates minor impurities, while electronic-grade demands high distillation efficiency. Lab methods prioritize control over yield.
Safety and Handling in Production
Dichloromethylsilane is highly reactive, flammable (flash point -26°F), and moisture-sensitive, releasing corrosive HCl gas upon hydrolysis. Industrial processes use sealed, inert systems with emissions controls for HCl and chloromethane. Waste minimization includes recycling silicon residues and treating effluents. In labs, handle under fume hoods with protective gear; environmental considerations focus on minimizing volatile organic compound emissions.
Dichloromethylsilane is chiefly produced industrially as a secondary product in the Müller–Rochow Direct Process, with laboratory methods offering alternatives via chlorination or Grignard reactions for specialized needs. Its role in organosilicon synthesis underscores the importance of efficient production and handling. While industrial routes dominate for scale, lab methods provide flexibility for research and custom applications.
Looking for a reliable bulk supplier of Dichloromethylsilane (CAS 75-54-7)?
Aure Chemical provides Premium Dichloromethylsilane (CAS 75-54-7) raw materials.
View our Dichloromethylsilane (CAS 75-54-7) product page
