Trimethylsilyl Triflate vs Trimethylsilyl Chloride: Which Silylating Agent Performs Better?
If you're into organic chemistry, you've probably encountered silylating agents—these are the molecular bodyguards that slap a silicon-based shield onto functional groups like alcohols, amines, or carboxylic acids, keeping them safe during reactions. Today, we're pitting two heavyweights against each other: Trimethylsilyl Triflate (TMSOTf) and Trimethylsilyl Chloride (TMSCl). Both introduce the trusty trimethylsilyl (TMS) group, but they do it in very different styles.
Drawing from expert sources in the field, including insights from Gelest's technical library, scientific reviews on ScienceDirect, and even real-talk discussions from chemists on Reddit, we'll break down their chemical profiles, reactivity showdown, practical applications, pros and cons, and finally, declare a winner (spoiler: it might depend on your setup). Whether you're a student prepping for exams or a researcher optimizing protocols, we'll keep things straightforward—no need for a lab coat to follow along!
What Are These Agents, Anyway?
Let's start with the basics. Both TMSOTf and TMSCl are reagents used to attach the TMS group (that's (CH₃)₃Si-) to your molecule, creating silyl ethers or similar derivatives that are more stable, volatile, or easier to handle in subsequent steps.
Trimethylsilyl Chloride (TMSCl): This is the classic choice, a colorless liquid with the formula (CH₃)₃SiCl. It's been around since the mid-20th century and is super common in labs because it's affordable and versatile.
Trimethylsilyl Triflate (TMSOTf): The "upgraded" version, with the formula (CH₃)₃SiOSO₂CF₃. Here, the chloride is replaced by a trifluoromethanesulfonate (triflate, or OTf) group, which makes it punchier. It's a bit newer on the scene but has gained fans for tough jobs.
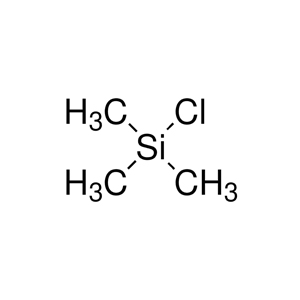
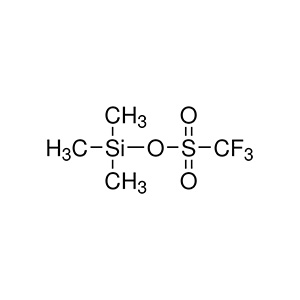
Think of TMSCl as your reliable sedan—gets you there steadily—while TMSOTf is the sports car, zipping through reactions but requiring a steadier hand.
Reactivity and Mechanism: Speed vs. Control
Here's where the rubber meets the road. Reactivity is all about how quickly and efficiently these agents do their job, and it's tied to their leaving groups (the part that gets kicked off during the reaction).
Mechanism Basics: Both work via nucleophilic substitution. Your substrate (like an alcohol, R-OH) attacks the silicon atom, displacing the leaving group—Cl⁻ for TMSCl or OTf⁻ for TMSOTf. The result? R-OSi(CH₃)₃ plus a byproduct (HCl or HOTf, triflic acid).
TMSCl's Style: It's moderately reactive and often needs a helping hand. You typically add a base like triethylamine (Et₃N) or pyridine to neutralize the HCl byproduct, which can be corrosive and mess up your reaction if not handled. Without a base, it might drag on or cause side reactions. In polar solvents like DMF, it can get a boost from the solvent acting as a Lewis base. Kinetic studies show it's slower for sterically hindered or secondary alcohols, with half-lives in the minutes to hours range depending on catalysts.
TMSOTf's Edge: Whoa, this one's a beast—studies show it can react up to 6.7 × 10⁸ times faster than chlorides in similar setups! The triflate is an excellent leaving group (thanks to its low bond energy and high electronegativity), so it often doesn't need catalysts. It silylates most alcohols in high yields, even tricky ones, and the triflic acid byproduct is easily trapped with a tertiary amine. However, this speed can backfire—it's so reactive it might generate destructive silylium ions (TMS⁺) that chew up sensitive substrates.
Winner in Reactivity? TMSOTf takes the crown for sheer speed, but TMSCl offers better control, especially for selective silylation (e.g., primary over secondary alcohols, with selectivity ratios up to 140:1 vs. lower for triflates).
Applications: Where They Shine in the Real World
These agents aren't just theoretical—they're workhorses in synthesis and analysis.
TMSCl Applications: Perfect for standard protection in multi-step organic synthesis, like in pyrimidinones or amino acids for GC/MS analysis. It's great for making volatile derivatives for chromatography, where the TMS group boosts solubility and thermal stability. In labs, it's often used with hexamethyldisilazane (HMDS) as a catalyst. Real example: Silylating sterically hindered alcohols or in NMR studies for silylotropic rearrangements.
TMSOTf Applications: Ideal for high-stakes, fast reactions, like protecting alcohols in complex molecules where time is critical. It's stellar for uncatalyzed silylations in apolar solvents and shines in kinetic resolutions or chemoselective protections. In battery or materials science, analogs are used for stable electrolytes, but for silylation, it's top for high-yield conversions. Case in point: Silylating phosphonates or sensitive substrates where milder agents fail, though chemists warn it might be overkill.
Both are deprotected easily (e.g., with dilute HCl or fluoride sources like TBAF), but TMS groups from either are labile—great for temporary shields but not long-term.
Pros and Cons: The Balanced View
| Aspect | TMSCl Pros | TMSCl Cons | TMSOTf Pros | TMSOTf Cons |
| Reactivity | Controllable with catalysts | Slower, needs base to neutralize HCl | Extremely fast, often catalyst-free | Too reactive; risk of side reactions |
| Handling | Affordable, widely available | HCl byproduct corrosive, needs venting | Triflic acid easily trapped | More expensive, moisture-sensitive |
| Selectivity | High (primary > secondary) | Less effective for hindered groups | High yields even for tough cases | Lower selectivity due to speed |
| Applications | Versatile for GC/MS, general synth | Labile products, hydrolysis-prone | Ideal for rapid, high-yield protections | Not suited for acid-sensitive substrates |
| Safety | Standard lab precautions | Acidic fumes | Cleaner byproducts | Strong acid byproduct if not trapped |
From Reddit chemists: For phosphonate silylation, skip both if you want neutral conditions—go milder like HMDS—but if you need power, TMSOTf with excess base buffers the acidity.
Which One Performs Better?
It boils down to your needs. If you're after speed and high yields for challenging substrates, TMSOTf is the champ—it's like upgrading to turbo mode in your reactions. But for everyday lab work, cost-effectiveness, and better selectivity, TMSCl holds its ground as the reliable go-to. Ultimately, the "better" agent depends on your substrate, conditions, and tolerance for byproducts. Experiment safely, and always check MSDS for handling!

