Why is Methyltrichlorosilane Hydrophobic?
Methyltrichlorosilane (MTS), with the chemical formula CH₃SiCl₃ (often abbreviated as CH₃Cl₃Si in structural notation), is an organosilicon compound renowned for its hydrophobic (water-repelling) characteristics. Hydrophobicity refers to a material's tendency to resist wetting by water, which arises from its inability to form favorable interactions with water molecules. In the case of MTS, this property is not merely a surface phenomenon but is deeply rooted in its molecular architecture, self-assembly behavior, and inherent chemical stability. Below, I break down these factors in detail, drawing directly from the structural and functional insights provided.
Chemical Structure: Low Surface Energy and Reduced Hydrogen Bonding
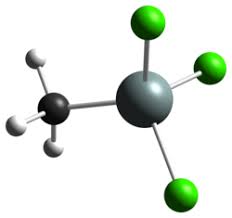 At the core of MTS's hydrophobicity is its molecular structure, which features a central silicon atom bonded to one methyl group (CH₃) and three chlorine atoms (Cl). This asymmetric arrangement creates a molecule with a highly polar silicon-chlorine backbone but a non-polar methyl terminus, resulting in an overall low surface energy.
At the core of MTS's hydrophobicity is its molecular structure, which features a central silicon atom bonded to one methyl group (CH₃) and three chlorine atoms (Cl). This asymmetric arrangement creates a molecule with a highly polar silicon-chlorine backbone but a non-polar methyl terminus, resulting in an overall low surface energy.
Low Surface Energy Mechanism: Surface energy is a measure of the cohesive forces at a material's interface. Water has a high surface energy (approximately 72 mN/m at 25°C) due to strong hydrogen bonding between its molecules. In contrast, MTS's Si-Cl bonds are relatively non-polar and exhibit weak intermolecular forces, leading to a surface energy typically below 30 mN/m when applied as a coating. This mismatch makes it energetically unfavorable for water to spread on MTS-treated surfaces; instead, water beads up into droplets with a high contact angle (often >90°, indicating hydrophobicity, and up to 150° for superhydrophobic variants).
Impeded Hydrogen Bonding: Water's polarity allows it to form extensive hydrogen bonds (O-H···O interactions), which stabilize liquid water and promote wetting on compatible surfaces. The CH₃ group in MTS is hydrophobic by nature, as its C-H bonds do not participate in hydrogen bonding. The Si-Cl bonds, while polar, are shielded by the bulky chlorine atoms, which sterically hinder water molecules from approaching the silicon core closely enough to form Si-OH or Cl···H-O interactions. As a result, MTS surfaces lack the hydrogen-bond donor or acceptor sites needed for water adhesion, causing water to remain as discrete droplets rather than wetting the surface.
This structural design mimics natural hydrophobic systems, such as lotus leaves, where low-energy alkyl chains repel water. In practical terms, the tri-chlorinated silicon provides reactive sites for covalent attachment to substrates (via hydrolysis to Si-OH groups), anchoring the hydrophobic methyl layer permanently.
Molecular Arrangement: Formation of a Dense Hydrophobic Barrier
Beyond its individual molecular properties, MTS's hydrophobicity is amplified by how its molecules self-assemble on surfaces, forming a compact, ordered layer that physically excludes water.
Self-Assembly and Layer Formation: When MTS is applied to a substrate (e.g., via vapor deposition or solution coating), it undergoes partial hydrolysis in the presence of trace moisture: CH₃SiCl₃ + 3 H₂O → CH₃Si(OH)₃ + 3 HCl. The resulting silanol (CH₃Si(OH)₃) groups then condense with surface hydroxyls (e.g., on glass or metal oxides), forming Si-O-Si covalent bonds. Unreacted or partially hydrolyzed MTS molecules orient themselves such that the non-polar CH₃ groups point outward, creating a densely packed monolayer or multilayer film. This orientation is driven by thermodynamic minimization of energy: polar Si-OH groups anchor to the substrate, while hydrophobic CH₃ tails expose a low-energy exterior.
Barrier Effect Against Water Intrusion: The dense packing of MTS molecules—often achieving monolayer thicknesses of 1-2 nm—creates a continuous hydrophobic barrier. Water molecules, with their tetrahedral geometry and ~0.275 nm diameter, face steric repulsion from the protruding CH₃ groups (van der Waals radius ~0.2 nm) and cannot penetrate the layer. This results in the Cassie-Baxter wetting regime, where air pockets trapped beneath water droplets further enhance repellency. For instance, on treated glass, this arrangement can achieve water contact angles exceeding 110°, preventing fogging or corrosion in humid environments.
This molecular-level organization ensures long-term durability, as the covalent anchoring resists mechanical abrasion or environmental degradation, making MTS ideal for protective coatings.
Chemical Properties: Stability and Resistance to Aqueous Reactions
MTS's hydrophobicity is sustained by its chemical inertness toward water under controlled conditions, preventing degradation that could expose hydrophilic sites.
Hydrolytic Stability: While MTS is moisture-sensitive and hydrolyzes to release HCl (a corrosive by-product), this reaction is self-limiting on hydrophobic surfaces. The initial hydrolysis forms a passivating silanol layer that quickly condenses, sealing the surface against further water ingress. The Si-Cl bonds are labile but selective: they react preferentially with surface silanols rather than bulk water, maintaining the hydrophobic integrity. This controlled reactivity contrasts with fully hydrophilic materials like silica, which swell and dissolve in water.
Thermal and Oxidative Resistance: MTS-derived films exhibit thermal stability up to 200-300°C
 and resistance to oxidation, preserving the low-surface-energy CH₃ groups. In aqueous environments, the absence of reactive functional groups (e.g., no carbonyls or amines) minimizes nucleophilic attacks by water, ensuring the hydrophobic layer remains intact over time. This stability is crucial for applications in harsh conditions, such as marine coatings or biomedical implants, where prolonged water exposure could otherwise compromise performance.
and resistance to oxidation, preserving the low-surface-energy CH₃ groups. In aqueous environments, the absence of reactive functional groups (e.g., no carbonyls or amines) minimizes nucleophilic attacks by water, ensuring the hydrophobic layer remains intact over time. This stability is crucial for applications in harsh conditions, such as marine coatings or biomedical implants, where prolonged water exposure could otherwise compromise performance.pH and Environmental Resilience: The hydrophobic layer buffers against pH fluctuations; acidic conditions from HCl release are localized and do not propagate wetting. This resilience extends to ionic solutions, where MTS repels saline water effectively, reducing biofouling or corrosion.
Summary: Interplay of Structure, Arrangement, and Stability
In essence, the hydrophobic properties of methyltrichlorosilane arise from a synergistic interplay: its low-energy chemical structure (CH₃SiCl₃) disrupts hydrogen bonding and wetting; its molecular arrangement forms an impermeable barrier; and its chemical stability ensures longevity. These attributes not only explain its water-repellent behavior but also underpin its broad applications in coatings (e.g., anti-fog glass), resins (e.g., silicone elastomers), and crosslinking agents (e.g., in adhesives). By mimicking and enhancing natural hydrophobicity at the nanoscale, MTS enables innovative solutions in materials science, from sustainable textiles to advanced electronics, with ongoing research exploring bio-inspired modifications for even greater efficacy.
Looking for a reliable bulk supplier of Methyltrichlorosilane?
Aure Chemical provides Premium Methyltrichlorosilane raw materials.
View our Methyltrichlorosilane product page
