How Trimethylsilyl Triflate Is Used as a Powerful Silylation and Catalytic Agent in Organic Synthesis
Trimethylsilyl Triflate's structure and reactivity stem from its unique combination of a trimethylsilyl group (Si(CH₃)₃) and a triflate leaving group (CF₃SO₃⁻), making it a highly electrophilic silylating agent. The formula CF₃SO₃Si(CH₃)₃ features a silicon atom bonded to the strongly electron-withdrawing triflate, which enhances its Lewis acidity and promotes rapid silyl transfer.
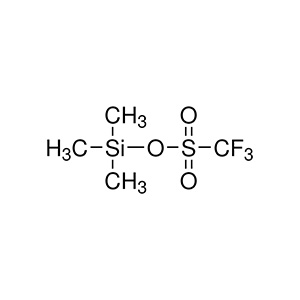 As a Lewis acid catalyst, TMSOTf coordinates with electron-rich atoms like oxygen in carbonyls, forming activated complexes that facilitate nucleophilic attack. For instance, in carbonyl activation, TMSOTf generates silyl enol ethers or oxocarbenium ions, lowering reaction barriers for additions or rearrangements. Its mechanism often involves the generation of a silylium ion equivalent (R₃Si⁺), which is more reactive than traditional silyl halides due to the excellent leaving group ability of triflate (OTf⁻).
As a Lewis acid catalyst, TMSOTf coordinates with electron-rich atoms like oxygen in carbonyls, forming activated complexes that facilitate nucleophilic attack. For instance, in carbonyl activation, TMSOTf generates silyl enol ethers or oxocarbenium ions, lowering reaction barriers for additions or rearrangements. Its mechanism often involves the generation of a silylium ion equivalent (R₃Si⁺), which is more reactive than traditional silyl halides due to the excellent leaving group ability of triflate (OTf⁻).
Compared to other silylating agents like TMSCl (trimethylsilyl chloride) or TMSI (trimethylsilyl iodide), TMSOTf offers higher reactivity under milder conditions, often at room temperature without strong bases. TMSCl requires bases like triethylamine for activation, while TMSOTf's intrinsic acidity allows catalyst-free operations in many cases. However, TMSOTf is more moisture-sensitive, hydrolyzing to form HF and silanol byproducts, necessitating anhydrous conditions.
The TMSOTf mechanism as a Lewis acid involves coordination to substrates, promoting reactions with high selectivity and efficiency, making it a preferred choice for complex molecule synthesis.
Major Applications in Organic Synthesis
TMSOTf's applications in organic synthesis are diverse, leveraging its dual role as a silylating agent and catalyst. Its high reactivity enables transformations that are challenging with less potent reagents, often achieving yields above 80–90% under optimized conditions.
Silylation of Alcohols and Phenols
TMSOTf is extensively used for protecting hydroxyl groups via silylation, converting alcohols and phenols to trimethylsilyl ethers. This protection is  crucial in multi-step syntheses to prevent unwanted reactions. The process involves adding TMSOTf to the alcohol in the presence of a base like 2,6-lutidine, forming the silyl ether rapidly at low temperatures.
crucial in multi-step syntheses to prevent unwanted reactions. The process involves adding TMSOTf to the alcohol in the presence of a base like 2,6-lutidine, forming the silyl ether rapidly at low temperatures.
For example, in carbohydrate chemistry, TMSOTf catalyzes the silylation of polyols in one-pot procedures, enabling selective protection and subsequent acetylation. Yields can reach 90–95%, with the reagent's acidity promoting clean conversions without byproducts like HCl from TMSCl. This application is vital in pharmaceutical synthesis, where protecting groups must be installed and removed selectively.
Glycosylation and Carbonyl Activation
In glycosylation reactions, TMSOTf acts as a promoter for forming glycosidic bonds, activating glycosyl donors like trichloroacetimidates or thioglycosides. It generates glycosyl cations under mild conditions, facilitating stereoselective couplings with acceptors. For instance, in the synthesis of oligosaccharides, TMSOTf enables efficient O-glycosylation of phenols, with yields often exceeding 80% and good α/β selectivity.
For carbonyl activation, TMSOTf converts ketones and aldehydes to silyl enol ethers, which serve as nucleophiles in aldol or Mukaiyama reactions. This is particularly useful in asymmetric synthesis, where TMSOTf's catalytic amounts (1–5 mol%) drive high-efficiency transformations. In peptide or natural product synthesis, it activates amides for N-silylation, aiding in deprotection or rearrangement steps.
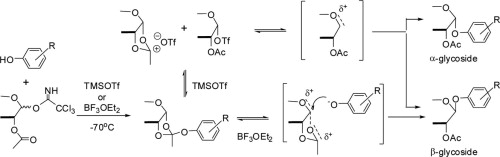
TMSOTf-catalyzed glycosylation reaction scheme.
Friedel-Crafts and Acylation Reactions
TMSOTf is a potent catalyst for Friedel-Crafts acylation and alkylation, promoting electrophilic aromatic substitutions with high regioselectivity. It activates acyl chlorides or anhydrides to form acylium ions, enabling reactions at room temperature with yields up to 90%. For example, in the synthesis of aryl ketones, TMSOTf outperforms traditional AlCl₃ by avoiding moisture sensitivity and allowing catalytic use (0.1–1 mol%).
In addition reactions, such as the cyanosilylation of acetals, TMSOTf catalyzes the addition of TMSCN, forming nitriles efficiently. This application extends to OLED material synthesis, where TMSOTf facilitates precise functional group introductions.
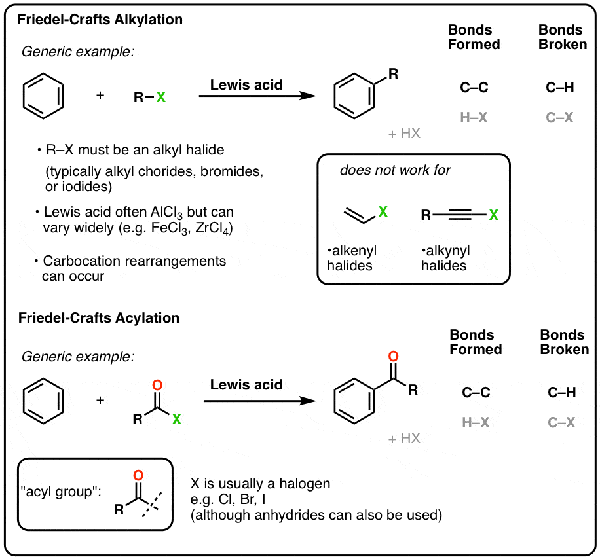
Friedel-Crafts acylation example with TMSOTf.
Polymer and Fine Chemical Synthesis
TMSOTf catalyzes polymerization reactions, such as the ring-opening of siloxanes or the formation of polysilanes, aiding in specialty polymer production. In fine chemicals, it promotes dehydration or rearrangement in steroid or alkaloid synthesis, offering mild conditions for sensitive substrates.
Advantages Over Other Reagents
TMSOTf's advantages make it a preferred choice over other silylating agents like TMSCl or TMSOTs (trimethylsilyl tosylate). Its superior reactivity stems from the excellent leaving group ability of triflate, allowing reactions at lower temperatures and with catalytic amounts, reducing waste and improving yields.
Compared to TMSCl, which generates HCl and requires stoichiometric bases, TMSOTf produces neutral byproducts and operates under milder, base-free conditions, minimizing side reactions. Versus TMSI, TMSOTf is less corrosive and more selective, avoiding iodide-related issues in halogen-sensitive syntheses.
| Property | TMSOTf | TMSCl |
Reactivity | Very high | Moderate |
Reaction Temperature | Low (RT or below) | Higher |
By-Products | Minimal (neutral) | HCl formation |
Typical Purity | ≥99% | ≥98% |
These benefits translate to higher efficiency in large-scale manufacturing, where TMSOTf's selectivity reduces purification steps and costs.
Handling, Storage, and Safety
TMSOTf is a corrosive, moisture-sensitive liquid that requires careful handling. It reacts violently with water, producing HF and silanol, so use in a fume hood with PPE including gloves, goggles, and protective clothing is mandatory. In case of skin contact, rinse immediately with water and seek medical attention.
For storage, keep under inert gas (nitrogen or argon) in sealed containers at 2–8°C to prevent hydrolysis. Shelf life is typically 1–2 years under proper conditions. Reference the SDS for detailed hazards, including its potential to cause severe burns and respiratory irritation.
With its outstanding catalytic activity, selectivity, and versatility, Trimethylsilyl Triflate continues to be indispensable in modern organic synthesis. Aurechem provides reliable supply with guaranteed purity and quality assurance under ISO-certified production systems. Whether for silylation, glycosylation, or Friedel-Crafts reactions, TMSOTf's powerful performance drives innovation in pharmaceuticals, materials, and beyond.

