Diglycolamine vs MEA: Which Amine to Choose?
In the world of industrial chemistry, amines are like the unsung heroes of cleaning up dirty gases—think of them as specialized sponges that soak up unwanted acidic contaminants like hydrogen sulfide (H₂S) and carbon dioxide (CO₂) from natural gas streams, refinery emissions, or even chemical synthesis processes. These compounds are crucial for "sweetening" sour gases, making them safer and more usable, much like removing the bitter rind from a fruit to reveal the sweet inside. Among the popular choices are Diglycolamine (DGA) and Monoethanolamine (MEA), both effective at scrubbing acids but with distinct personalities in terms of performance, cost, and suitability. DGA might be the sturdy, efficient option for tough jobs, while MEA is the budget-friendly workhorse for everyday tasks. This blog post aims to break down their differences to help you pick the right amine for your process, whether you're in gas treatment, flue gas scrubbing, or beyond.
Overview of Diglycolamine (DGA)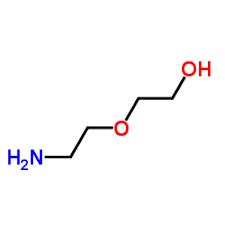
Diglycolamine, or DGA, has the chemical formula C₄H₁₁NO₂ and a structure that looks like HOCH₂CH₂OCH₂CH₂NH₂—a chain with an alcohol group on one end and an amine on the other, allowing it to grab onto acids from multiple angles.
It's a clear liquid with a boiling point of around 221°C, making it less volatile (less likely to evaporate like water on a hot day), high solubility in water, and low vapor pressure. With a molecular weight of 105.14 g/mol, it's heftier than some counterparts.
DGA shines in natural gas sweetening and acid gas removal, especially for H₂S and CO₂, and in specialty treatments where you need minimal corrosion and vapor loss—imagine it as a gentle but thorough cleaner that doesn't leave streaks or evaporate quickly. Advantages include high absorption capacity (it can hold more gas per gallon of solution), low vapor pressure to reduce losses, good thermal stability, and the ability to snag extras like COS and mercaptans. However, its higher viscosity (thicker like honey) can make pumping trickier, and it comes at a higher cost.
Overview of Monoethanolamine (MEA)
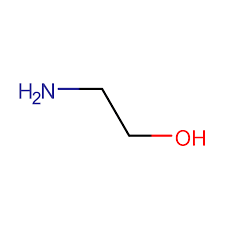 Monoethanolamine, or MEA, boasts a simpler formula, C₂H₇NO, and structure: HOCH₂CH₂NH₂—a short chain with both alcohol and amine groups close together, making it highly reactive like a quick-draw magnet for acids.
Monoethanolamine, or MEA, boasts a simpler formula, C₂H₇NO, and structure: HOCH₂CH₂NH₂—a short chain with both alcohol and amine groups close together, making it highly reactive like a quick-draw magnet for acids.
It has a lower molecular weight of 61.08 g/mol, a boiling point of about 170°C (more volatile, evaporating faster like alcohol in hand sanitizer), and excellent water solubility.
MEA is a staple in acid gas removal and flue gas treatment in refineries and power plants, acting as a rapid responder to scrub out H₂S and CO₂. Its advantages? Low cost, well-established tech that's like the reliable old truck in your garage, and high reactivity for quick cleanups. On the flip side, it has a higher corrosion rate (eating away at equipment like rust on metal), degrades in the presence of CO₂ and oxygen, and requires more energy for regeneration—like reheating leftovers that take forever in the microwave.
Comparative Analysis: DGA vs MEA
To make the choice clearer, here's a side-by-side comparison table highlighting key factors:
| Property / Factor | Diglycolamine (DGA) | Monoethanolamine (MEA) |
| Molecular Weight | Higher (105.14 g/mol) | Lower (61.08 g/mol) |
| Volatility | Low (BP 221°C) | Higher (BP 170°C) |
| Corrosiveness | Lower | Higher |
| CO₂/H₂S Absorption Efficiency | High (prefers CO₂, good for COS/mercaptans) | Moderate (rapid reaction, good for low H₂S) |
| Regeneration Energy | Lower net (despite high heat of reaction, offset by capacity) | Higher (high heat of reaction ~825 BTU/lb CO₂) |
| Thermal Stability | Better (less degradation with contaminants) | Moderate (degrades with CO₂/O₂) |
| Cost | Higher | Lower |
| Common Use | Natural gas sweetening, refinery gases with mercaptans | Flue gas treatment, low-pressure total removal |
This table shows DGA as the more robust option for efficiency and stability, while MEA wins on affordability and simplicity. For instance, DGA's higher concentration in solutions (50-70% vs. MEA's 10-20%) means lower circulation rates, like using a concentrated detergent that cleans more with less product, but its preference for CO₂ over H₂S can be a quirk in mixed gas streams. MEA, meanwhile, reacts faster overall but pays for it with more corrosion and energy demands.
Selection Criteria: How to Choose
Choosing between DGA and MEA is like picking between a premium vacuum and a basic one—it depends on your needs. If your process demands low corrosion, minimal solvent loss, and handling of extras like COS or mercaptans, go with DGA; it's ideal for high-acid-gas partial pressures or cold climates where its low freezing point shines. On the other hand, if cost and straightforward operation are priorities, especially for low-pressure systems needing total acid gas removal, MEA is your economical pick.
Key factors to weigh:
Type of acid gas: DGA for CO₂-heavy or mercaptan-laden streams; MEA for rapid H₂S pickup at low levels.
Gas composition and pressure: DGA handles high pressures better without overheating.
Energy costs: MEA's higher regeneration energy could spike bills.
System compatibility: MEA's corrosiveness might require stainless steel upgrades.
Practical Case Examples
To see this in action, consider a natural gas sweetening plant with high H₂S concentrations—DGA would excel here, absorbing contaminants efficiently with less circulation and handling COS as a bonus, like a multi-tool fixing multiple issues at once. In contrast, for flue gas desulfurization in a refinery, MEA's low cost and proven track record make it the go-to, despite needing more energy to regenerate, akin to using a reliable but fuel-hungry vehicle for daily commutes.
In summary, DGA offers superior stability, lower corrosion, and better handling of tricky contaminants for natural gas sweetening, while MEA remains the economical, reactive choice for flue gas treatment and basic scrubbing. No amine is universally "best"—it hinges on your application's economics, operating conditions, and gas profile. Looking ahead, emerging amine blends combining the strengths of DGA and MEA (or others like MDEA) are gaining traction for even more optimized performance. By evaluating your specific needs, you can select the amine that keeps your processes running smoothly and cost-effectively.
Related Articles
Looking for a reliable bulk supplier of Diglycolamine?
Aure Chemical provides Premium Diglycolamine (CAS 929-06-6).
View our Diglycolamine product page
