How Diglycolamine Is Produced: Manufacturing Process
Diglycolamine (DGA) is primarily produced through ethoxylation of monoethanolamine with ethylene oxide or amination of diethylene glycol with ammonia, using reactors and distillation for purification to achieve 99%+ purity. Key equipment includes stirred tanks and vacuum columns, with strict controls on temperature and pressure to ensure yield while managing safety risks like exothermic reactions—vital for consistent quality in gas treatment applications.
Introduction
Ever wondered how a versatile chemical like diglycolamine (DGA), also known as 2-(2-aminoethoxy)ethanol or aminoethoxyethanol, gets from raw materials to the tankers that deliver it to industries? This compound, with its amine and alcohol groups, is a workhorse in sectors like natural gas processing, where it scrubs out acidic gases like a diligent housekeeper removing stains from a carpet. It's also key in making corrosion inhibitors, surfactants, and even polyurethane resins—think of it as the glue holding together products from detergents to protective coatings.
Understanding DGA's production isn't just academic; it's crucial for engineers ensuring supply chain reliability, managers watching costs, and regulators enforcing safety. Poor process control can lead to impurities that foul up downstream uses or, worse, safety incidents. In this post, we'll walk through the manufacturing journey, from feedstocks to final packaging, using everyday analogies to demystify the tech.
Chemical & Physical Basics
DGA's chemical formula is C₄H₁₁NO₂, with CAS number 929-06-6. Imagine it as a chain: an amino group (NH₂) linked to an ethoxy chain ending in a hydroxyl (OH), like a flexible rope with hooks at both ends for easy reactions.
Physically, it's a clear, slightly viscous liquid—think honey but less sticky—with a mild ammonia-like odor. It melts at -12.5°C and boils at 221°C, has a density of about 1.056 g/mL, and mixes easily with water and alcohols, much like sugar dissolving in tea.
Customers demand high specs: purity ≥99%, water content <0.1%, color <15 APHA, and amine value around 560 mg KOH/g. These ensure it performs without gumming up systems.
| Property | Value |
| Molecular Weight | 105.14 g/mol |
| Boiling Point | 221°C |
| Melting Point | -12.5°C |
| Density | 1.056 g/mL at 20°C |
| Solubility | Miscible in water, alcohols |
| pH (10% solution) | ~11.5 |
Feedstocks & Raw Materials
DGA starts with simple building blocks. For the ethoxylation route, key feedstocks are monoethanolamine (MEA, NH₂-CH₂-CH₂-OH) and ethylene oxide (EO, a reactive ring molecule). MEA provides the amine base, while EO adds the ether link—like snapping Lego pieces together.
In the amination route, diethylene glycol (DEG, HO-CH₂-CH₂-O-CH₂-CH₂-OH) and ammonia (NH₃) are used, with DEG as the backbone and NH₃ replacing an OH group. Catalysts like nickel or copper-chromite speed things up.
Purity is key: EO must be >99.9% to avoid side reactions, stored cold under nitrogen to prevent explosions. Ammonia needs low water content. Materials are handled in stainless steel tanks to dodge corrosion, with inventory managed via just-in-time to minimize hazards.
Common Synthesis Routes — Overview
Multiple paths lead to DGA, chosen based on available raw materials and plant setup. Think of it as different recipes for the same cake—some quicker, others cheaper.
Route A: Ethoxylation – MEA reacts with EO in a base-catalyzed process: NH₂-CH₂-CH₂-OH + C₂H₄O → NH₂-CH₂-CH₂-O-CH₂-CH₂-OH. Conditions: 100-150°C, moderate pressure, homogeneous catalyst like KOH. Pros: high yield (90%+), clean product; cons: EO handling risks, potential oligomers.
Route B: Amination – DEG + NH₃ → DGA + H₂O, over heterogeneous catalysts like Raney nickel at 200-250°C, 100-200 bar. Pros: uses cheaper DEG; cons: higher energy, catalyst deactivation.
Route C: Other Methods – Like BCME hydrogenation, but niche due to toxic intermediates. Lab-scale for specialties.
Typical Process Flow
Production is like a assembly line: start with prep, react, clean up.
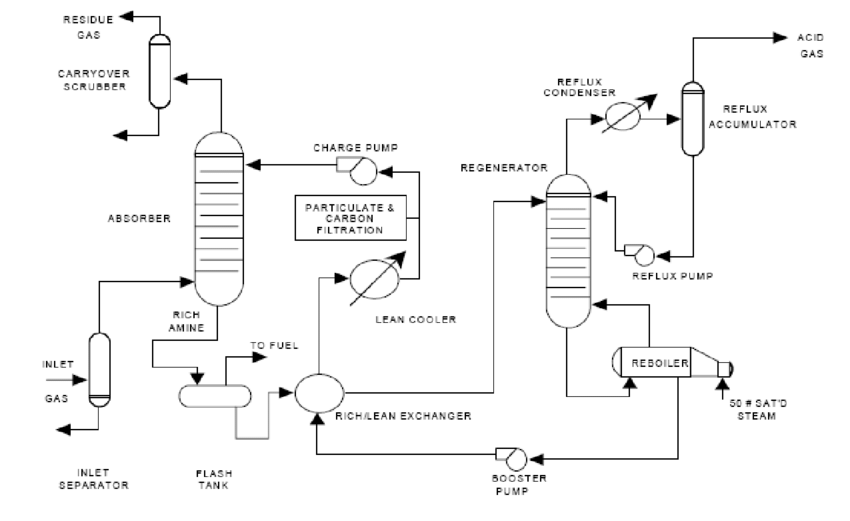
Feed Preparation: Dry and filter MEA/EO or DEG/NH₃ to remove impurities.
Reaction: In a continuous stirred tank reactor (CSTR), mix under controlled temp/pressure. Exothermic—like a baking soda volcano—so cooling coils prevent runaway.
Workup: Neutralize, quench, filter out catalysts.
Separation: Fractional distillation removes lights (unreacted EO/NH₃) and heavies.
Purification: Vacuum distillation hits high purity, stripping water/ethers.
Polishing: Activated carbon for color, final filters.
Controls: Stoichiometry (1:1 ratio), residence time (hours), monitor pH to avoid byproducts.
Equipment & Materials of Construction
Reactors are CSTRs or tubular for continuous flow, with agitation like a blender. Distillation uses packed columns under vacuum. Heat exchangers manage temps, filters handle solids.
Materials: 316 stainless steel resists corrosion; nickel alloys for EO areas. Linings prevent amine attacks.
Purification & Quality Control
Impurities like MEA, triethylene glycol, or water are zapped via distillation. Tests: GC for volatiles, Karl Fischer for water, titration for amine value.
| Impurity | Limit | Method |
| Water | <0.1% | Karl Fischer |
| MEA | <0.5% | GC |
| Color | <15 APHA | Spectrophotometry |
Safety, Environmental & Regulatory
Hazards: EO's flammability/explosivity (like a pressure cooker gone wrong), amine corrosivity/toxicity. Controls: Nitrogen blanketing, relief valves, scrubbers for vents.
Wastes: Aqueous streams treated biologically, catalysts recycled. Regs: OSHA exposure limits, EPA for VOCs.
Scale-up & Economics
Lab to plant: Shift batch to continuous for efficiency, tackle heat transfer. Costs: EO dominates (60%), energy for distills. Improve via recycles.
Quality Assurance, Traceability & Packaging
Batch records track everything, COAs certify. Store cool/dry in drums/IBCs, shelf life 2 years.
Troubleshooting & Common Problems
Low yield: Check catalyst activity; refresh if fouled.
High color: Add carbon polishing; trace metals from feeds.
Runaway temp: Calibrate sensors; add inhibitors.
Impurities: Adjust stoichiometry; extend distillation.
Applications & Downstream Uses
DGA shines in gas sweetening, needing low impurities for efficiency, plus synthesis of inhibitors tying back to pure feeds.
Environmental Sustainability & Alternatives
Go green with bio-based EO, catalyst recycling, energy-efficient distills. Alternatives: Other amines, but DGA balances eco-footprint.
Conclusion & Recommendations
DGA production blends chemistry and engineering for a vital industrial player. For next steps: Test lab routes, pilot scale, vet suppliers on purity/safety. Dive deeper with patents for innovations.
Related Articles
Looking for a reliable bulk supplier of Diglycolamine?
Aure Chemical provides Premium Diglycolamine (CAS 929-06-6).
View our Diglycolamine product page
