Applications of Lithium Triflate (CAS 33454-82-9) in Lithium Batteries: Conductivity, Stability, and Advantages
Lithium Triflate (LiCF₃SO₃, CAS 33454-82-9), also known as lithium trifluoromethanesulfonate, is a versatile organometallic salt widely recognized for its role in lithium-ion batteries (LIBs) and organic synthesis. In battery applications, it serves as a key component in electrolytes, offering high chemical reactivity, excellent solubility in non-aqueous solvents, and superior ionic conductivity. This article focuses on its contributions to LIBs, emphasizing enhancements in conductivity, stability, and overall performance advantages, while drawing on its physicochemical properties to explain these benefits.
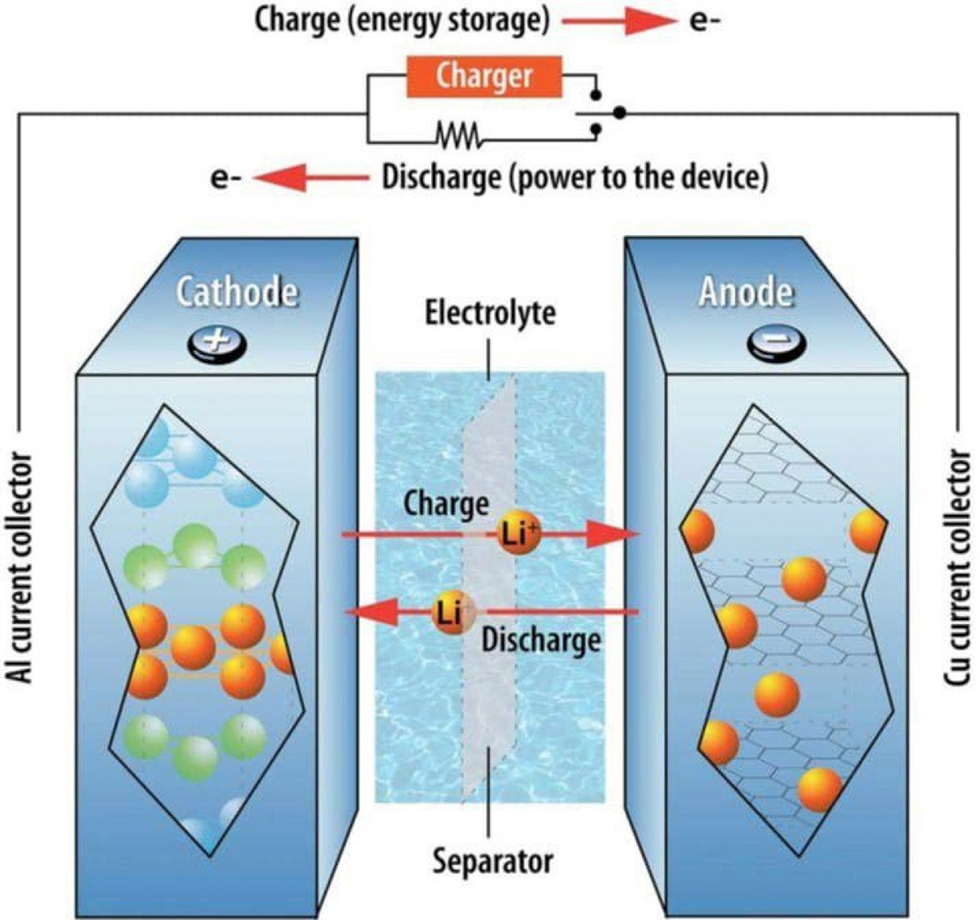
Electrolyte – Enabling ions transport within a Li-ion battery (Image Source: etn.news)
Enhancing Electrolyte Conductivity in Lithium-Ion Batteries
One of the primary applications of lithium triflate in LIBs is improving electrolyte conductivity, which is crucial for efficient ion transport and battery performance. When incorporated into electrolytes, lithium triflate can elevate conductivity to levels around 10⁻³ S/cm, facilitating faster charge-discharge cycles and higher power output. Studies on polymer electrolytes doped with lithium triflate show that increasing its concentration boosts both AC and DC conductivities to orders of 10⁻² S/cm, enhancing lithium-ion mobility and reducing internal resistance.
In gel polymer electrolytes or solid-state systems, lithium triflate acts as a salt additive that promotes ion dissociation and transport. For instance, in systems combining lithium triflate with polymers like polyethylene oxide (PEO), the salt's high lithium-ion transference number (approximately 0.4) ensures efficient lithium migration, minimizing polarization and improving battery efficiency. This makes it particularly useful in high-rate applications, such as electric vehicles, where rapid ion conduction is essential.
Superior Stability: Thermal, Electrochemical, and Chemical Resilience
Lithium triflate's stability is a standout feature, making it ideal for demanding battery environments. It exhibits excellent thermal stability, with decomposition temperatures typically exceeding 200°C, allowing it to withstand high operating temperatures without degrading. This property ensures reliable performance in battery systems exposed to heat, reducing the risk of thermal runaway.
From an electrochemical perspective, lithium triflate offers a broad electrochemical window of 4–5 V, enabling compatibility with high-voltage cathodes like lithium nickel manganese cobalt oxide (NMC) or lithium iron phosphate (LFP). This wide window minimizes redox decomposition, enhancing cycle life and safety. In pseudoconcentrated electrolytes, it contributes to high ionic conductivity while maintaining stability, supporting high-voltage LIB chemistries.
Chemically, its solubility in non-aqueous solvents like propylene carbonate (>5 mol/L at 25°C) promotes uniform dispersion, preventing phase separation and ensuring long-term stability. High-purity lithium triflate (>99.9%) further reduces side reactions, extending battery lifespan by minimizing impurities that could lead to degradation.
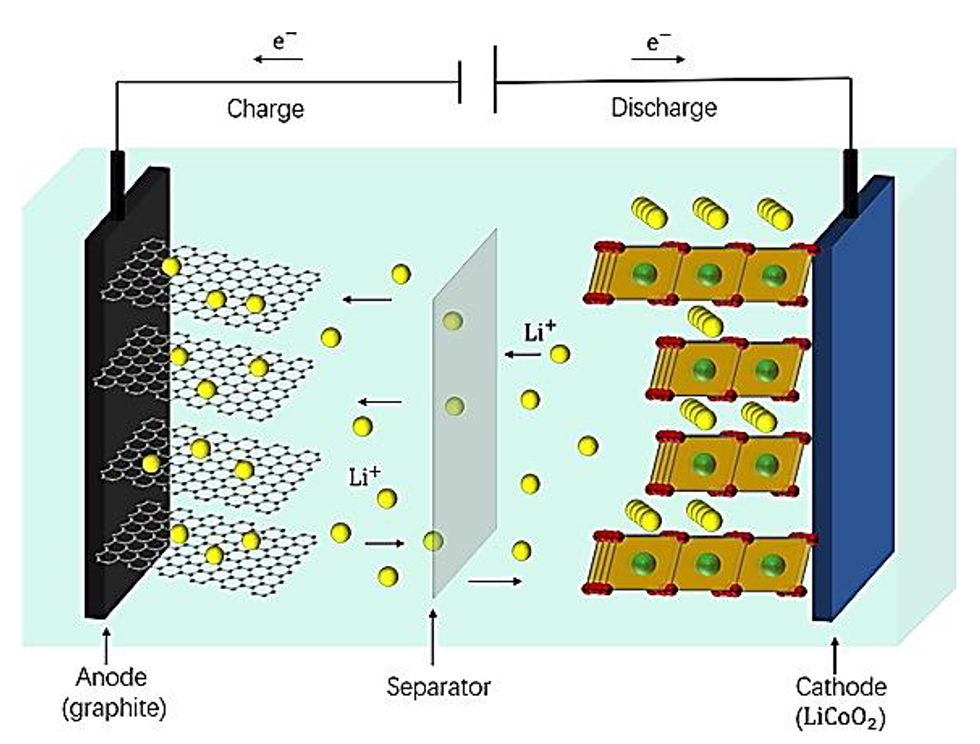
The Impact of Polymer Electrolyte Properties on Lithium-Ion Batteries (Image Source:mdpi.com)
Key Advantages in Lithium Battery Applications
Lithium triflate provides several advantages over traditional salts like lithium hexafluorophosphate (LiPF₆), including better thermal and chemical stability, which reduces safety risks in high-temperature or abusive conditions. Its use in low-temperature electrolytes enhances performance in cold environments, where ion conductivity often drops, making it suitable for applications in aerospace or electric vehicles in varying climates.
In multifunctional solvent designs, lithium triflate enables high-voltage operations, boosting energy density while maintaining stability. Its role in polymer electrolytes further improves mechanical flexibility and safety in solid-state batteries, addressing issues like dendrite formation. Overall, these attributes contribute to longer cycle life, higher efficiency, and safer battery systems.
Conclusion: The Role of Molecular Structure in Battery Performance
The efficacy of lithium triflate in LIBs stems from its molecular structure, where the highly electronegative fluorine atoms enhance ion dissociation and stability. This structural feature provides selectivity and reactivity, making it a preferred additive for next-generation batteries. As research advances, lithium triflate's applications are poised to expand, supporting sustainable energy solutions with improved conductivity, stability, and performance advantages.
Related Articles
Looking for a reliable bulk supplier of Lithium Triflate?
Aure Chemical provides Premium Lithium Triflate (CAS 33454-82-9).
View our Lithium Triflate product page
