Applications of Boron Trifluoride Acetonitrile Complex
Boron Trifluoride Acetonitrile Complex, or BF₃·CH₃CN (CAS 420-16-6), is a handy chemical tool in the world of organic chemistry, acting as a Lewis acid 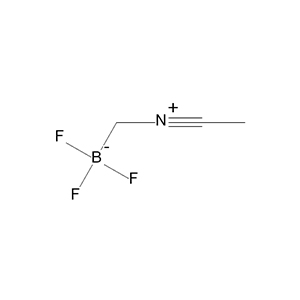 that's like a master key unlocking tough reactions. This colorless to light yellow liquid combines the power of boron trifluoride (BF₃) with acetonitrile for better stability and handling, making it a go-to reagent and catalyst in labs and industries. From crafting new drugs to building advanced materials, BF₃·CH₃CN plays a crucial role in efficient, selective synthesis, helping chemists create complex molecules with precision.
that's like a master key unlocking tough reactions. This colorless to light yellow liquid combines the power of boron trifluoride (BF₃) with acetonitrile for better stability and handling, making it a go-to reagent and catalyst in labs and industries. From crafting new drugs to building advanced materials, BF₃·CH₃CN plays a crucial role in efficient, selective synthesis, helping chemists create complex molecules with precision.
Overview of Boron Trifluoride Acetonitrile Complex
Chemical Properties
With the molecular formula BF₃·C₂H₃N and a molar mass of 108.86 g/mol, BF₃·CH₃CN is a colorless or light yellow transparent liquid that's miscible with water and various organic solvents—think of it dissolving as easily as sugar in coffee. It's stable under normal conditions but moisture-sensitive, often supplied as a solution with 15-17% BF₃ content.
Reactivity
As a Lewis acid, it eagerly accepts electron pairs from other molecules, kickstarting reactions like a spark igniting a fire. The acetonitrile ligand stabilizes BF₃, making it compatible with a range of reagents without the volatility issues of pure BF₃ gas. This setup allows for controlled catalysis in sensitive environments.
Applications in Organic Synthesis
As a Lewis Acid Catalyst
BF₃·CH₃CN excels in Friedel-Crafts reactions, where it helps attach groups to aromatic rings—imagine it as a matchmaker introducing substituents to benzene molecules for acylation (adding acyl groups) or alkylation (adding alkyl chains). Its solubility and selectivity outperform some other Lewis acids, reducing side reactions and improving yields in fine chemical production.
Cyclization Reactions
In forming cyclic compounds, crucial for pharmaceutical synthesis, BF₃·CH₃CN promotes ring closures like in heterocycle formation—similar to folding a paper chain into a loop. It's used in creating drug intermediates, offering clean, efficient paths without excessive byproducts, as seen in various patented processes.
Polymerization and Material Science
For polymerization, it initiates chain growth in resins or polymers, acting like a starter pistol for monomer linking. In material science, it aids cross-linking for durable coatings or adhesives, relevant in industries needing robust, high-performance materials.
Other Organic Transformations
Beyond these, it's involved in nitration (adding nitro groups), ether formation, and halogenation—facilitating bonds like a skilled welder joining metals. Its versatility extends to advanced syntheses in agrochemicals and specialty chemicals.
Advantages of Using BF₃·CH₃CN
High Selectivity: Targets specific reaction sites, minimizing waste like a precision laser.
Easy Handling: Liquid form is safer and more convenient than gaseous BF₃.
Stability: Predictable reactivity in various solvents, enhancing control.
Versatility: Compatible with diverse transformations, boosting efficiency.
| Advantage | Description |
| Selectivity | Reduces side products for cleaner yields. |
| Handling | Liquid state simplifies dosing and storage. |
| Stability | Maintains activity without rapid decomposition. |
| Reactivity | Strong Lewis acidity for fast reactions. |
Handling and Safety Considerations
BF₃·CH₃CN is corrosive and can release toxic fumes, so handle with care—like dealing with concentrated vinegar on steroids. Use PPE including gloves, goggles, and respirators in well-ventilated areas. Store in cool, dry conditions in sealed containers. Always refer to the Material Safety Data Sheet (MSDS) for detailed guidelines.
Boron Trifluoride Acetonitrile Complex (CAS 420-16-6) is a versatile catalyst powering organic synthesis through Friedel-Crafts, cyclization, polymerization, and more, benefiting pharmaceuticals, fine chemicals, and polymer production. Its advantages in selectivity and handling make it indispensable for efficient catalysis. Boron Trifluoride Acetonitrile Complex (CAS 420-16-6) remains an essential reagent for chemists seeking efficient and selective catalytic solutions.
Related Articles
Looking for a reliable bulk supplier of Boron Trifluoride Acetonitrile Complex?
Aure Chemical provides Premium Boron Trifluoride Acetonitrile Complex CAS 420-16-6.
View our Boron Trifluoride Acetonitrile Complex product page
