The Curious Case of Xylenes: Why o-Xylene's Melting Point is Low But Its Boiling Point is High
Have you ever wondered why two very similar molecules can behave so differently? Take o-xylene (ortho-Xylene) and p-xylene (para-Xylene, for example. They have the same chemical formula, C₈H₁₀, but their melting and boiling points are worlds apart. It's a classic chemistry puzzle, and the answer lies in their unique molecular structures.
 |  |
Melting Point: A Tale of Symmetry
The key to understanding the difference in melting points is molecular symmetry and how it affects solid-state packing.
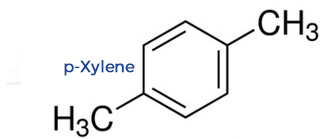
p-Xylene: The Tidy Molecule
Para-xylene's two methyl groups are at opposite ends (1,4 positions) of the benzene ring, making the molecule highly symmetrical.
This symmetry allows para-xylene molecules to pack together very efficiently and tightly in a crystal lattice.
To break this neat, orderly structure and melt the solid, a significant amount of energy is required, resulting in a higher melting point of 13°C.
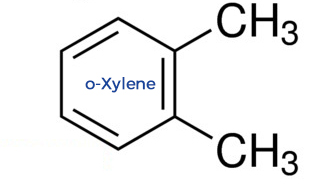
o-Xylene: The Awkward Fit
In contrast, ortho-xylene's two methyl groups are right next to each other (1,2 positions), giving the molecule an asymmetrical shape.
This lack of symmetry prevents the molecules from packing as tightly in the solid state.
As a result, the intermolecular forces in the solid are weaker, and less energy is needed to melt it, leading to a much lower melting point of -25°C.
In short: Melting point is mainly determined by molecular symmetry and crystal packing efficiency. Highly symmetrical molecules typically have higher melting points.
Boiling Point: The Strength of Intermolecular Forces
When it comes to boiling points, the story changes completely. Boiling is a liquid-to-gas phase transition, and it’s governed by the strength of the intermolecular forces holding the liquid molecules together.
o-Xylene: The Stickier Molecule
Due to its asymmetrical structure, ortho-xylene has a significant molecular dipole moment.
The close proximity of the methyl groups also increases the contact area between molecules, strengthening London dispersion forces.
These factors combine to create stronger intermolecular forces, which means more energy is needed to separate the molecules and turn the liquid into a gas. This leads to a higher boiling point of 144°C.
p-Xylene: The Less Sticky Molecule
Para-xylene's symmetrical structure results in a much smaller dipole moment and weaker intermolecular forces.
Consequently, less energy is required to overcome these forces and reach the boiling point, which is a lower 138°C.
In short: Boiling point is determined by the strength of intermolecular forces, including dipole-dipole interactions and dispersion forces. Asymmetrical molecules often have higher boiling points.
The Key Takeaway
This fascinating difference between ortho-xylene and para-xylene beautifully illustrates a core principle in chemistry: a molecule's properties are a direct result of its structure. Melting point is dictated by how well molecules pack together in the solid state, while boiling point is controlled by the strength of the forces between molecules in the liquid state.

