Production of Dimethyl Sulfoxide via the Methanol + Carbon Disulfide Process
Dimethyl sulfoxide (DMSO, CAS 67-68-5) is a versatile polar aprotic solvent widely used in pharmaceuticals, electronics, polymers, and chemical industries for its excellent solvency, low toxicity, and high boiling point. One of the traditional methods for its production is the methanol + carbon disulfide process, which is particularly prevalent in regions like China due to its cost-effectiveness and use of readily available raw materials. This process involves synthesizing dimethyl sulfide (DMS) as an intermediate from methanol and carbon disulfide, followed by oxidation to DMSO. This article details the reaction principle, process steps, catalysts, conditions, equipment, purification, safety considerations, and advantages, drawing from patents and industry sources.
Overview of the Process
The methanol + carbon disulfide process is a two-stage method: first, the synthesis of DMS from methanol (CH₃OH) and carbon disulfide (CS₂), and second, the oxidation of DMS to DMSO ((CH₃)₂SO). This route is favored for its simplicity and lower raw material costs compared to other methods like the dimethyl sulfate or wood pulp by-product processes. Global DMSO production capacity exceeds 100,000 MT/year, with this method contributing significantly in Asia. The process is energy-intensive but yields high-purity DMSO suitable for industrial and pharmaceutical grades after refinement.
Reaction Principle
The overall process can be summarized as follows:
Synthesis of Dimethyl Sulfide (DMS):
Methanol reacts with carbon disulfide in the presence of a catalyst to form DMS and by-products like hydrogen sulfide (H₂S) and other sulfides.
The primary reaction is: 2 CH₃OH + CS₂ → (CH₃)₂S + CO₂ + H₂S (simplified; actual mechanism involves intermediate thioesters or thiocarbamates). However, the exact stoichiometry may vary, and some sources indicate involvement of desulfurization steps.
Oxidation of DMS to DMSO:
DMS is oxidized using an oxidant like nitrogen dioxide (NO₂), hydrogen peroxide (H₂O₂), or air/oxygen, often with catalysts.
Reaction: (CH₃)₂S + [O] → (CH₃)₂SO, where [O] represents the oxygen from the oxidant. Over-oxidation can produce dimethyl sulfone ((CH₃)₂SO₂), so conditions are controlled to favor DMSO selectivity.
Process Steps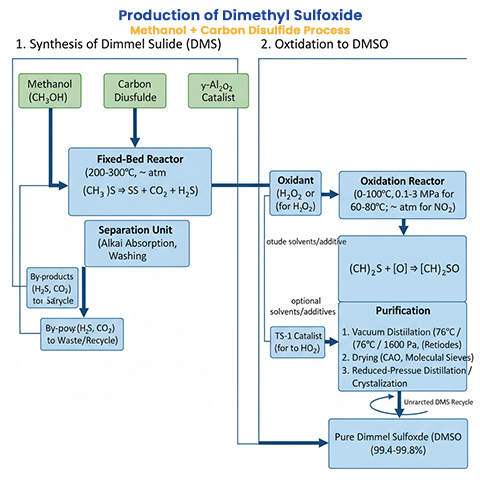
Synthesis of DMS
Raw Materials: Methanol and carbon disulfide in a molar ratio typically 2:1 or adjusted for optimal yield.
Catalyst: γ-Al₂O₃ (gamma-alumina) or Al₂O₃, which facilitates the reaction by providing acidic sites for activation.
Conditions: The reaction occurs under heating (typically 200-300°C, though exact temperatures vary), often in a fixed-bed reactor with vapor-phase contact. Pressure is atmospheric or slightly elevated.
Equipment: Tubular reactor or fixed-bed catalytic reactor for continuous operation.
By-Products Management: By-products like H₂S and CO₂ are separated via desulfurization (e.g., using alkali absorption) and alkaline washing.
Yield: Up to 85-90% for DMS, depending on catalyst efficiency.
Oxidation to DMSO
Raw Materials: DMS from the previous step, with oxidants such as NO₂ (traditional) or H₂O₂ (modern, for better selectivity).
Catalyst: For H₂O₂ oxidation, titanium-silicon molecular sieves (e.g., TS-1 with MFI structure) at 1-100 wt% in the catalyst formulation. For NO₂, no catalyst is typically needed, but gas-liquid phase reactors are used.
Conditions:
For NO₂ oxidation: 60-80°C in gas-liquid phase, atmospheric pressure.
For H₂O₂ oxidation: 0-100°C (preferably 20-80°C), pressure 0.1-3 MPa (preferably 0.1-1.5 MPa gauge). Molar ratio DMS:H₂O₂ = 1:0.1-2 (preferably 1:0.2-1). Mass ratio DMS:catalyst = 0.1-100:1.
Equipment: Batch reaction kettles or continuous fixed-bed/slurry-bed reactors. For fixed-bed, weight hourly space velocity of DMS is 0.1-10000 h⁻¹.
Solvents and Additives: Optional solvents like water or methanol (mass ratio 1-1000:1 to DMS); surfactants (e.g., ammonium salts) at low concentrations to enhance rate.
Purification
Crude DMSO is purified by vacuum distillation, collecting the fraction at 76°C / 1600 Pa (12 mmHg), keeping temperature below 90°C to prevent disproportionation to dimethyl sulfone and DMS. Drying agents like CaO, BaO, or molecular sieves are used, followed by reduced-pressure distillation or partial crystallization. Purity can reach 99.4-99.8%, with yields up to 98% selectivity in optimized oxidation.
Process Considerations
Yield and Efficiency: Overall yields are 85-98% for DMSO, with high oxidant utilization (up to 99%) in catalytic oxidation.
By-Product Management: H₂S and sulfides are recovered or treated; NO₂ oxidation may produce NOx emissions, requiring scrubbers.
Catalyst Regeneration: Titanium-silicon sieves can be recycled up to 60 times with minimal activity loss.
Safety and Handling
DMSO is hygroscopic and can form explosive mixtures with certain oxidants; handle CS₂ (toxic, flammable) in ventilated systems. Use PPE and monitor for H₂S leaks. Environmental controls include wastewater treatment for sulfides.
The methanol + carbon disulfide process offers an economical route to DMSO via DMS intermediate, with γ-Al₂O₃ for synthesis and advanced catalysts like TS-1 for oxidation, achieving high yields under mild conditions. Advantages include cost savings and catalyst reusability, though it requires careful by-product management. Modern optimizations focus on green oxidants like H₂O₂ for sustainability.
Looking for a reliable bulk supplier of Dimethyl Sulfoxide (DMSO)?
Aure Chemical provides high-purity DMSO raw materials for the pharmaceutical, polymer, and chemical industries.
View our DMSO product page
