Preparation of Diethyl Ether in the Laboratory
Reaction Principle
Main Reaction
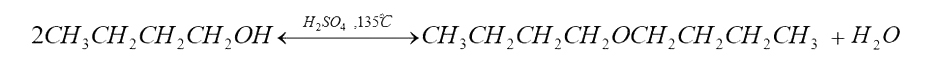
Side Reaction
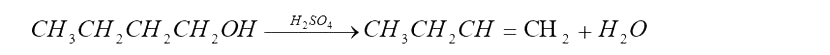
There are two common methods for obtaining a better yield from reversible reactions:
① Use an excess of inexpensive raw materials;
② Immediately remove one of the reaction products from the reaction zone after it is formed.
The first method is not applicable in this experiment, so the second method is used to quickly remove the water formed from the reaction zone. Therefore, the method of removing the water formed during the reaction is used.
Experimental apparatus
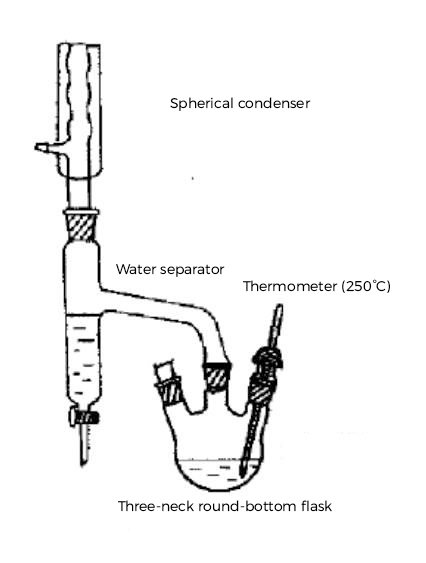 | 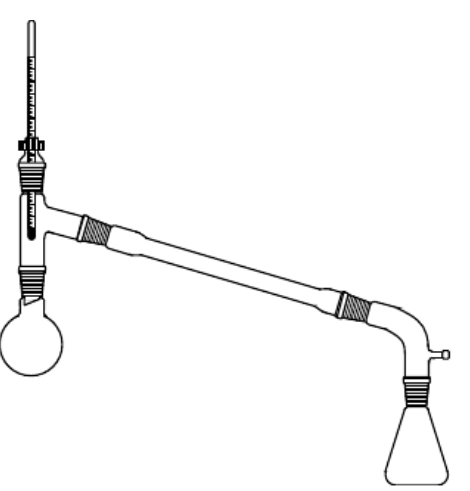 |
| Reaction Apparatus | Product Collection |
Experimental Apparatus and Reagents
Apparatus: Round-bottom flask, thermometer (250°C), straight-tube water condenser, water separator or Dean-Stark trap, conical flask, alcohol lamp, iron stand, separatory funnel.
Reagents: n-Butanol (A.R.), concentrated Sulfuric Acid, anhydrous Calcium Chloride (A.R.).
| Reagents | Molecular Weight | mp ℃ | bp℃ | Density | PH | Solubility / g·mL⁻¹ | ||
| H₂O | Ethanol | Ether | ||||||
| n-Butanol | 74.12 | -89.8 | 118 | 0.89 | 7 | Soluble | Soluble | Soluble |
| Diethyl Ether | 130.23 | -98 | 142.4 | 0.76 | 5.2 | Slightly soluble | Soluble | Soluble |
| H₂SO₄ | 98.08 | 10.38 | 340 | 1.83 | –1 | Soluble | Soluble | Soluble |
Physical constants of reagents
Experimental Procedure

Synthesis of Dibutyl Ether
Take a 100mL three-neck round-bottom flask, electric heater, asbestos gauze, condenser, thermometer (note: the thermometer should be inserted below the liquid surface), and a Dean-Stark trap. Assemble the apparatus in the order of "bottom-up, left-to-right": round-bottom flask, Dean-Stark trap, and a spherical condenser. Before assembly, the Dean-Stark trap must be checked for leaks. After assembly, fill the Dean-Stark trap with water and then drain 2 mL from the bottom. The volume of water should be measured with a graduated cylinder.
Take 31 mL (approximately 0.37 mol) of n-butanol and add it to a small beaker. Then, slowly add 4.5 mL (approximately 0.08 mol) of concentrated sulfuric acid to it (note: during the addition, stir with a glass rod to prevent localized high concentration of sulfuric acid from oxidizing the n-butanol, which would turn the solution pink).
Pour the well-mixed, colorless, and transparent n-butanol and concentrated sulfuric acid solution from the side opening of the three-neck flask into the 100mL flask. Add 1-3 boiling chips (too many may adsorb a large amount of the product during post-processing, affecting the yield). Adjust the position of the thermometer (note: the thermometer should be inserted below the liquid surface but not touching the flask wall).
Turn on the electric heater. Start with high heat to allow the vapor to reflux in the condenser, then reduce the heat to maintain the reflux and separation of water in the trap. When the Dean-Stark trap is completely filled with water, drain 1.8 mL from the bottom. Continue the reaction until the trap is again full of water or the reaction temperature in the three-neck flask reaches 134-136°C (reaction time is about 1.5 hours). Immediately stop heating and remove the heat source. If heating continues, the reaction solution will turn black, and a significant amount of alkene by-products will form.
Liquid-Liquid Separation and Drying
After the reaction mixture has cooled to room temperature, pour it into a separatory funnel containing 50mL of water (note: the product must be poured into the water layer in the separatory funnel; the order cannot be reversed. This sequence ensures that concentrated sulfuric acid is added to the aqueous solution). Shake thoroughly. After standing, separate the organic layer. The upper layer is the crude product, and the lower layer is the sulfuric acid layer, which should be discarded.
Wash the crude product layer sequentially with 30mL of water, 15mL of 5% sodium hydroxide solution, 15mL of water, and 15mL of saturated calcium chloride solution (a total of 4 washes and separations).
Place the washed crude product in a dry narrow-necked bottle (e.g., a conical flask or an iodine flask). Add anhydrous calcium chloride to dry the product. Once the drying is complete, the crude product solution will become a clear liquid, and the calcium chloride will appear as dispersed particles.
Distillation
Transfer the dried product to a small distillation flask. Distill the product and collect the fraction boiling between 139-142°C.
Precautions:
When adding sulfuric acid, it must be done while stirring, and the rate of addition should not be too fast.
The volume of water to be drained from the Dean-Stark trap must be pre-calculated. The water level should be 3-5mm below the lower edge of the reflux branch of the trap to ensure that the alcohol can return to the reaction system in a timely manner to continue the reaction. The theoretical calculated volume of water to be removed in this experiment is 3.33 mL, so approximately 3.8 mL of water should be drained. However, draining 4 mL at once would cause the liquid level in the trap to drop too much, leaving too much n-butanol temporarily, which is not conducive to the reaction. Therefore, the procedure is to drain it in two steps: first drain 2 mL, and then drain another 1.8 mL when the trap is almost full again. Note: Do not drain the water as long as it is not returning to the reaction system.
The ideal temperature for preparing Dibutyl Ether is 130-140°C. However, this temperature is difficult to reach at the beginning of reflux because Dibutyl Ether can form an azeotrope with water (boiling point 94.1°C, 33.49% water). Additionally, a ternary azeotrope of Dibutyl Ether, water, and n-butanol can form (boiling point 90.6°C, 29.9% water, 34.6% n-butanol), and n-butanol can also form an azeotrope with water (boiling point 93°C, 44.5% water). Therefore, the reaction should be carried out at 100-115°C for half an hour before the temperature can exceed 130°C.
During the alkali washing process, do not shake the separatory funnel too vigorously; otherwise, an emulsion may form, making separation difficult. If an emulsion does form, a small amount of an electrolyte like table salt or water, or sonication can be used to help the layers separate.
n-Butanol is soluble in a saturated calcium chloride solution, while Dibutyl Ether is only slightly soluble.
The crude product layer can also be washed twice with 30mL of cold 50% sulfuric acid first, and then twice with 30mL of water. The 50% sulfuric acid can wash away the n-butanol from the crude product, but Dibutyl Ether is also slightly soluble, which will result in a slightly lower yield.
Looking for a reliable bulk supplier of Dibutyl Ether (CAS 142-96-1)?
Aure Chemical provides Premium Dibutyl Ether (CAS 142-96-1) raw materials.
View our Dibutyl Ether (CAS 142-96-1) product page
