Industrial Production Methods of Styrene Monomer
In the landscape of modern chemical industry, there is an unassuming liquid molecule whose unique structure underpins countless plastic products in our daily lives—from sturdy automotive components and lightweight appliance casings to the familiar foam packaging we encounter everywhere. That molecule is styrene. As the cornerstone of important polymers such as polystyrene, ABS plastic, and styrene-butadiene rubber, the synthesis process of Styrene Monomer is not only a rigorous science but also a continuously evolving industrial history.
This article will take you on a journey to explore the origins of this critical molecule. We will trace the primary production method of styrene—the ethylbenzene dehydrogenation process—analyze the underlying chemical principles and catalyst secrets, and understand how the industry balances efficiency and cost. Through this article, you will not only gain an understanding of the synthesis process of styrene but also appreciate the precision and ingenuity of the chemical industry.
What is Styrene
Styrene is an important organic compound with the chemical formula C₈H₈, a colorless to pale yellow oily liquid with a characteristic aromatic odor at room temperature.
Physical and Chemical Properties
Structure: The structure of styrene consists of a benzene ring (C₆H₅) and a vinyl group (-CH=CH₂).
CAS No.: 100-42-5.
Density: The density is approximately 0.906 g/mL at 20°C.
Boiling Point: The boiling point is approximately 145-146°C.
Flash Point: The flash point is approximately 31-32°C.
Applications
Styrene is a very important polymer, mainly used in the production of:
Polymers:Polystyrene;ABS plastic ( a terpolymer made from acrylonitrile, butadiene, and styrene monomers);Styrene-butadiene rubber (SBR);Styrene-acrylonitrile resin (SAN).
Industrial Applications: Styrene is also used in the manufacture of paints, inks, synthetic rubber, and other products. It has applications in the petrochemical, oil and gas, organic and inorganic chemical, and pharmaceutical industries.
Synthesis of Styrene
Let’s discuss the synthesis of styrene, the monomer in polystyrene. Styrene is a highly important polymer monomer, used not only for the synthesis of ethylbenzene but also for the production of ABS and styrene-butadiene rubber.
ABS Plastic
ABS is a te/rpolymer of acrylonitrile, butadiene, and styrene, first introduced in 1953. Among these three components, acrylonitrile provides the material with heat resistance and corrosion resistance, butadiene gives the material toughness, and styrene provides plasticity. The relative proportions of these three raw materials can be adjusted to meet various different requirements.
Sources of Ethylbenzene
Styrene can be synthesized through various methods, but the primary source remains ethylbenzene dehydrogenation. This has also made ethylbenzene another important aromatic hydrocarbon product alongside para-xylene. The sources of ethylbenzene are primarily twofold: first, ethylbenzene is produced during the cracking of alkanes, and this portion can be obtained through aromatic extraction. Additionally, since benzene has low value in aromatics and is produced in excess, it is commonly alkylated with ethylene to produce ethylbenzene. This has become the mainstream process following the significant increase in styrene demand.
In the early days, benzene alkylation reactions typically relied on FC alkylation reactions. This method required the use of highly 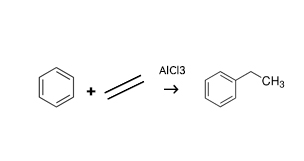 corrosive AlCl₃ as a catalyst, imposing stringent material selection requirements on equipment and pipelines. It was largely phased out after the 1970s. Currently, alkylation reactions generally use zeolite molecular sieve catalysts. The initial process employed the gas-phase method, where benzene and ethylene were reacted at 400°C and 2 MPa through a catalyst bed to produce the product. In the 1990s, the liquid-phase method was developed, increasing the reaction pressure to approximately 4 MPa to ensure the reaction occurred in the liquid phase, with the reaction temperature reduced to around 240°C. Lowering the reaction temperature suppresses side reactions and improves product yield.
corrosive AlCl₃ as a catalyst, imposing stringent material selection requirements on equipment and pipelines. It was largely phased out after the 1970s. Currently, alkylation reactions generally use zeolite molecular sieve catalysts. The initial process employed the gas-phase method, where benzene and ethylene were reacted at 400°C and 2 MPa through a catalyst bed to produce the product. In the 1990s, the liquid-phase method was developed, increasing the reaction pressure to approximately 4 MPa to ensure the reaction occurred in the liquid phase, with the reaction temperature reduced to around 240°C. Lowering the reaction temperature suppresses side reactions and improves product yield.
Ethylbenzene Dehydrogenation
After addressing the source of ethylbenzene, let us examine the dehydrogenation of ethylbenzene. The ethylbenzene dehydrogenation process first emerged in 1930. Over decades of improvements, major manufacturers introduced distinctive processes, including the UOP/Lummus method and the Fina/Badger method.
Dehydrogenation of ethylbenzene and side reactions
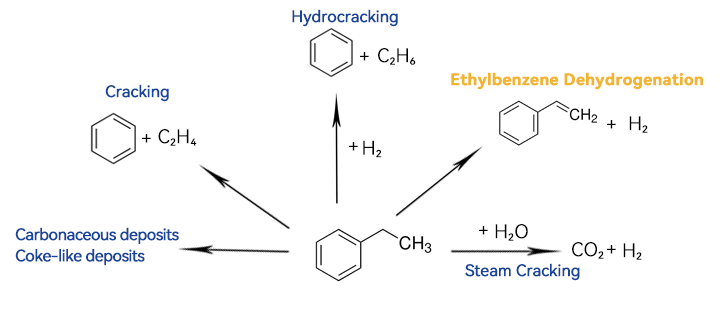
The ethylbenzene dehydrogenation reaction is an exothermic reversible reaction that involves an increase in reaction volume. Research indicates that higher temperatures are more favorable for increasing conversion rates, while the increase in reaction volume necessitates conducting the overall reaction under low pressure. However, we cannot directly reduce the reaction pressure because the reaction involves organic compounds and produces hydrogen gas. Operating under negative pressure could potentially draw oxygen into the reactor, leading to an explosion. Therefore, industrial processes often require the injection of steam to reduce the partial pressures of the reactants and products.
Of course, the cracking reaction is also influenced by a series of side reactions, with the most significant being the direct cracking of ethylbenzene into benzene at high temperatures. To suppress the cracking reaction, a catalyst is required. Catalysts typically use an oxide catalyst system, with Zn-based catalysts being used during the initial industrialization in the 1930s. After World War II, the common Fe-K-Cr ternary oxide system emerged, where iron oxide serves as the main catalyst, K₂O adjusts the catalyst's acidity to reduce coking, and Cr₂O₃ enhances the catalyst's thermal stability.
The reaction temperature is favorable for both ethylbenzene dehydrogenation and cracking, increasing reaction rate and conversion, but it is not conducive to improving selectivity, typically around 600°C. To ensure selectivity, the reaction requires a short residence time, which also results in low overall conversion rates. This necessitates separation of raw materials and products at the outlet and recycling of raw materials.
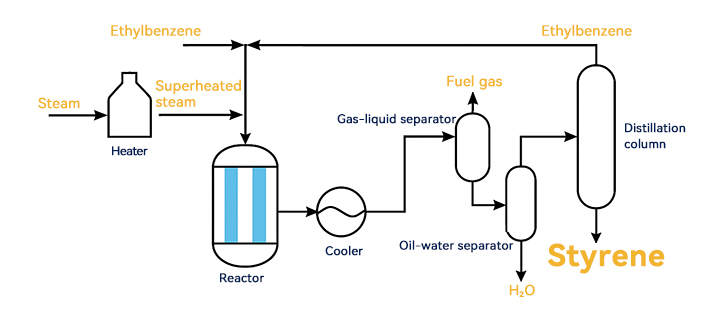
Ethylbenzene dehydrogenation process flow
Ethylbenzene dehydrogenation reactors typically use fixed-bed reactors. Due to the high-temperature requirements of the reaction, differences arise between isothermal external heating processes and adiabatic processes in terms of heating methods. The isothermal external heating process uses high-temperature flue gas to heat the reactor through heat exchanger tubes. The adiabatic process directly heats water vapor to 700°C to form superheated steam, which is introduced into the reactor to provide heat for the reaction. The adiabatic process has a simple equipment structure and does not require complex tube bundles to increase heat transfer area, making it the mainstream process for ethylbenzene dehydrogenation today.
Co-oxidation method for producing Styrene
In addition to the ethylbenzene dehydrogenation process, the Hakon method, also known as the co-oxidation method, is used in styrene production to produce a small amount of ethylene oxide as a byproduct. In this method, ethylbenzene first reacts with oxygen to form ethylbenzene hydrogen peroxide, which is then reacted with propylene to produce α-methylbenzyl alcohol and ethylene oxide. Subsequently, α-methylbenzyl alcohol undergoes dehydrogenation to form styrene. Additionally, there are processes such as ethylbenzene oxidation dehydrogenation and direct synthesis using toluene or benzene as raw materials; however, these processes are still under research.
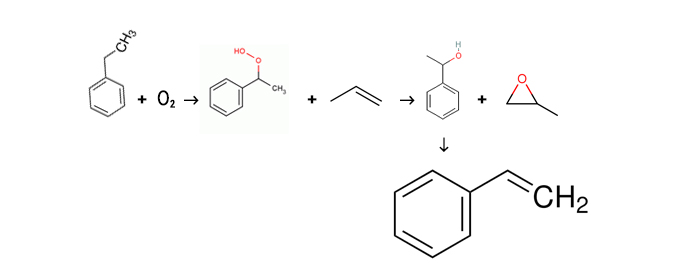
Co-oxidation method for producing styrene

