The Biological Reactions of Dimethyl Sulfoxide (DMSO): Properties, Mechanisms, and Medical Uses
Dimethyl sulfoxide (DMSO), a simple organic compound with the chemical formula (CH₃)₂SO, is a polar aprotic solvent renowned for its exceptional ability to dissolve both polar and nonpolar substances. First identified in the late 19th century as a byproduct of the kraft process in paper manufacturing, DMSO gained scientific attention in the mid-20th century for its remarkable biological properties. It is colorless, hygroscopic, and miscible with water, making it an ideal solvent in various applications. Biologically, DMSO stands out due to its rapid penetration through skin and cellular membranes without causing damage, allowing it to act as a carrier for drugs and other molecules into living tissues. This has led to its widespread use in medical treatments, veterinary care, and laboratory research, where it functions as an anti-inflammatory agent, analgesic, antioxidant, and cryoprotectant.
In medicine, DMSO is approved for specific conditions like interstitial cystitis and is incorporated into topical formulations for pain relief. Veterinarians employ it to treat injuries in animals, while researchers leverage its properties in cell culture, drug discovery, and cryopreservation. Its low toxicity and versatility have sparked ongoing investigations into its mechanisms, despite historical safety concerns that temporarily halted clinical trials. Today, DMSO's biological reactions continue to intrigue scientists, influencing everything from gene expression to tissue healing. But what makes DMSO so biologically active — and how does it interact with living cells? This article delves into its properties, mechanisms, effects, safety, and future potential, providing a comprehensive overview for those interested in its multifaceted role in biology and medicine.
What Is Dimethyl Sulfoxide (DMSO)?
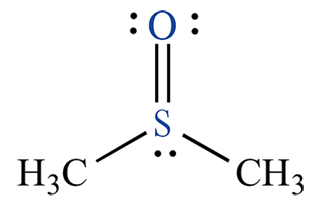 Dimethyl sulfoxide, commonly abbreviated as DMSO, is an organosulfur compound with the molecular formula (CH₃)₂SO. Its structure features a central sulfur atom bonded to two methyl groups and a double-bonded oxygen, classifying it as a sulfoxide. This configuration imparts high polarity, enabling DMSO to form hydrogen bonds with water and other molecules, which contributes to its excellent solubility profile. It is fully miscible with water, ethanol, acetone, and many organic solvents, but insoluble in nonpolar hydrocarbons like hexane.
Dimethyl sulfoxide, commonly abbreviated as DMSO, is an organosulfur compound with the molecular formula (CH₃)₂SO. Its structure features a central sulfur atom bonded to two methyl groups and a double-bonded oxygen, classifying it as a sulfoxide. This configuration imparts high polarity, enabling DMSO to form hydrogen bonds with water and other molecules, which contributes to its excellent solubility profile. It is fully miscible with water, ethanol, acetone, and many organic solvents, but insoluble in nonpolar hydrocarbons like hexane.
Industrially, DMSO originates as a byproduct of the kraft process used in wood pulping, where lignin is separated from cellulose using sulfur-containing compounds. This sustainable source has made it readily available for commercial applications since the 1950s. What sets DMSO apart is its unique solvent capabilities and rapid penetration through biological barriers, such as skin and cell membranes, without disrupting their integrity. This property stems from its ability to interact with lipids and proteins, facilitating the transport of solutes across these barriers.
Key characteristics of DMSO include:
Appearance and Odor: Colorless liquid with a mild, garlic-like odor (though pure DMSO is odorless; the scent arises from its metabolite, dimethyl sulfide).
Physical Properties: Boiling point of 189°C, melting point of 19°C, and highly hygroscopic nature, absorbing moisture from the air.
Solubility: Dissolves a wide range of substances, making it a versatile solvent in pharmaceuticals and research.
Toxicity Profile: Relatively low acute toxicity, with an oral LD50 in rats of 14,500 mg/kg, higher than many common solvents like ethanol.These traits underpin DMSO's biological relevance, positioning it as a tool for enhancing drug delivery and modulating cellular processes.
How DMSO Interacts with Biological Systems
DMSO's ability to cross biological membranes effortlessly is one of its most notable features, allowing it to enter cells and tissues rapidly after topical or systemic application. This penetration occurs without causing permanent damage to the membrane structure, distinguishing it from more disruptive solvents. The mechanism involves DMSO's interaction with the lipid bilayer of cell membranes, where it disrupts hydrogen bonding between water molecules and phospholipids, temporarily increasing membrane fluidity and permeability. Additionally, DMSO forms hydrogen bonds with polar groups on proteins and lipids, facilitating its diffusion across barriers like the stratum corneum of the skin.
At the cellular level, DMSO influences osmotic balance by acting as an osmolyte, helping cells maintain equilibrium in response to environmental stresses. It exhibits antioxidant behavior by scavenging free radicals, such as hydroxyl radicals, through its sulfur atom, which can donate electrons to neutralize reactive oxygen species (ROS). This protective effect is concentration-dependent and contributes to its anti-inflammatory properties.
Biochemically, even low concentrations of DMSO (e.g., 0.1%) induce significant changes in cellular processes, including over 2,000 differentially expressed genes affecting transcription, translation, and epigenetics like DNA methylation. These alterations are consistent across tissues, such as cardiac and hepatic cells, suggesting broad biological impacts. In vitro, DMSO can arrest the cell cycle at the G1 phase in lymphoid cells and promote differentiation in embryonic carcinoma cells into specialized types like cardiomyocytes. However, its pleiotropic effects necessitate careful controls in research to avoid confounding results.
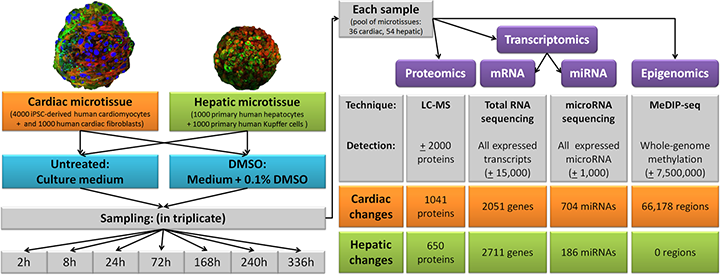
Infographic illustrating DMSO’s impact on cellular processes, including transcriptomics and epigenomics in human microtissues. (Image Source: nature.com)
Overall, DMSO's interactions stem from its chemical versatility, enabling it to modulate membrane dynamics, oxidative stress, and gene regulation in living systems.
Major Biological Reactions and Effects
Skin Penetration and Drug Transport
DMSO excels as a skin penetration enhancer, rapidly crossing the epidermis by solvating keratin and disrupting the lipid matrix. This allows it to carry small molecules (typically under 500 Da) into deeper tissues, though larger compounds may not penetrate effectively. Optimal concentrations for transport range from 45-90%, as seen in formulations like PENNSAID, where 45.5% DMSO aids diclofenac delivery for osteoarthritis pain. However, it does not enhance all drugs equally and can increase systemic absorption risks.
Pain Relief and Anti-inflammatory Activity
DMSO provides pain relief through mechanisms distinct from opioids like morphine, primarily by reducing inflammation via antioxidant effects and inhibiting prostaglandin synthesis. Unlike anesthetics, it does not block nerve conduction but modulates nociceptor activity. Clinical applications include topical use for arthritis, sprains, and injuries, where it alleviates pain without sedative side effects. Studies show relief in genitourinary disorders, with significant improvements in patient symptoms.
Antimicrobial and Antifungal Properties
At concentrations above 10%, DMSO inhibits bacterial and fungal growth by disrupting cell membranes and denaturing proteins. It synergizes with antibiotics, enhancing their penetration and efficacy against resistant strains. This property is utilized in compounded antifungals for nail infections, where DMSO facilitates drug delivery to the nail bed.
Diuretic and Osmotic Effects
DMSO acts as a diuretic by promoting water excretion and reducing edema through osmotic pressure modulation. It is particularly effective in treating cerebral edema and intracranial pressure, drawing fluid from swollen tissues. In veterinary medicine, intravenous DMSO helps manage brain and spinal swelling in animals.
Collagen Regulation and Tissue Healing
DMSO prevents excessive collagen deposition by inhibiting fibroblast activity, aiding in the treatment of fibrotic conditions like scleroderma. It promotes wound healing by reducing scar formation and enhancing tissue regeneration through anti-inflammatory pathways.
Immunomodulatory and Muscle Relaxation Effects
In animal models, DMSO reduces autoimmune antibody production and modulates immune responses by suppressing pro-inflammatory cytokines. It improves circulation by relaxing smooth muscles and vasodilating blood vessels, benefiting conditions like Raynaud's phenomenon.
Safety, Side Effects, and Toxicology
DMSO is generally safe at approved doses, with a high safety margin (LD50 of 14,500 mg/kg in rats). Common side effects include a garlic-like breath odor from dimethyl sulfide metabolism, skin irritation, itching, and headaches. Gastrointestinal reactions like nausea occur with oral use, and high concentrations can cause drowsiness or dizziness. It can enhance absorption of contaminants or drugs, potentially leading to interactions with blood thinners or sedatives.
Toxicology concerns include neurotoxicity in developing brains at low doses (0.3 mL/kg), causing apoptosis. The FDA approves it only for interstitial cystitis, with global regulations varying—e.g., Schedule 4 in Australia. Proper handling requires compatible gloves to prevent degradation and absorption.
Medical and Research Applications
Clinically, DMSO is FDA-approved for interstitial cystitis, providing symptomatic relief through anti-inflammatory effects on bladder lesions. It is used topically for arthritis pain in products like PENNSAID and as a cryoprotectant in cell banking, preventing ice crystal damage during freezing. In research, it enhances PCR by reducing DNA secondary structures and serves as a solvent in high-throughput screening, though its cellular effects require controls. Emerging uses include regenerative medicine for tissue preservation and drug delivery in nanomedicine.
Current Research and Future Perspectives
Recent studies (2024-2025) explore DMSO's biological applications, such as its interaction with human nerve endings in drug discovery, where it influences solubility but may alter protein structures. Research on metabolic disruptions in fish cells highlights oxidative stress responses at low concentrations. It mitigates cadmium-induced cytotoxicity by activating antioxidants and shows toxicity when combined with alkylating agents. Studies on capsid stability and RNA conformation suggest impacts on viral research. A 2025 systematic review affirms its efficacy in topical and intravesical uses, with focus on safety.
Future perspectives include DMSO derivatives for enhanced drug delivery and roles in pharmaceuticals, potentially in cancer therapy adjuvants or neuroprotective agents, as nanomedicine advances leverage its penetration properties.
DMSO's versatility as a solvent, penetrant, and modulator of biological processes has cemented its importance in medicine and research. From alleviating pain and inflammation to preserving cells and enhancing drug efficacy, its reactions with living systems offer profound benefits, tempered by careful safety considerations. Ongoing studies continue to uncover its potential, promising innovations in regenerative and pharmaceutical fields. For those in healthcare or science, exploring DMSO's applications further—perhaps through related resources on cryobiology or topical enhancers—could yield valuable insights into this enduring compound.
Looking for a reliable bulk supplier of Dimethyl Sulfoxide (DMSO)?
Aure Chemical provides high-purity DMSO raw materials for the pharmaceutical, polymer, and chemical industries.
View our DMSO product page
